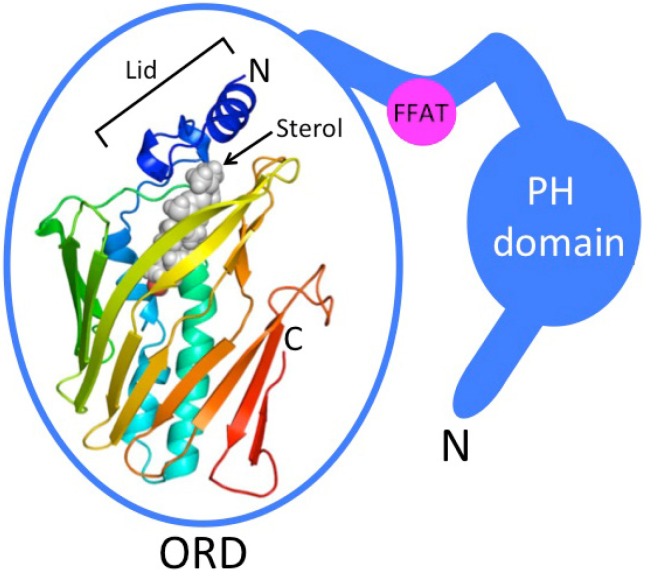
Figure 1.
Schematic structure of ORP proteins. ORD, OSBP-related ligand binding domain, represents human ORP1 ORD modeled by using the yeast Osh4p structure [33] as a template. Bound sterol, which stabilizes a closed conformation of a flexible ‘Lid’ structure, is depicted as a gray ball-model. β-strands are displayed as flat arrows and α-helical secondary structures as helices. While “Short” subtype ORPs, such as yeast Osh4p-Osh7p, consist of a mere ORD, the “Long” subtype family members (most mammalian ORPs belong to this category) carry an N-terminal extension (blue) with a pleckstrin homology (PH) domain interacting with phosphoinositides and a short peptide motif (two phenylalanines in an acidic tract, FFAT) that targets the ER.