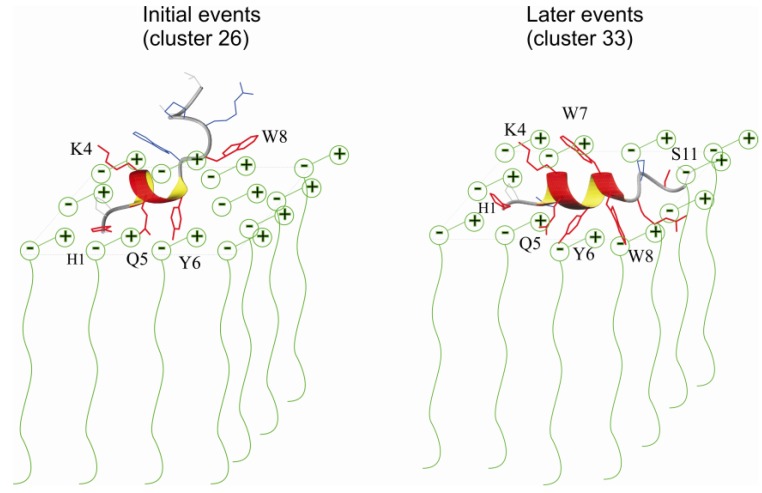
Figure 8.
Schematic representation of the proposed model of two events for PW2 association with DPC interface. In the left is a static representation of the association of a cluster 26 structure with the micelle. In red are the residues that are hydrogen bonded with DPC head group during simulation. Note that residues His 1, Lys 4, Gln 5 and Tyr 6 forms long-lived hydrogen bonds, while Trp 8 forms only short-lived hyrogens bonds. Cluster 26 structures interact superficially with the DPC head groups and the C-terminal residues are weakly bound. In the right we represent the association of a cluster 33 structure with DPC micelles. Note that that the helix was elongated if compared with cluster 26. This elongation was only possible in the simulation with free-DPC with spontaneous micelles formation. Our model assumes that this deeper association was only possible after micelle re-equilibration, event that happens in millisecond to second timescale. In red are the residues that are hydrogen bonded with DPC micelles. Note that most of the residues show long-lived hydrogen bonds. Number of intra-molecular hydrogen bonds (PW2-DPC) increased consistently when compared for the entire MD simulation as a function of the amino acid residue.