Table 1.
Optimization of the indium-mediated dehalogenation reaction a.
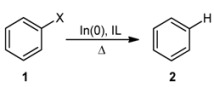
| |||||
|---|---|---|---|---|---|
| Entry | Solvent | X | Temperature (°C) | Yield b (%) 2 | Recovery b (%) 1 |
| 1 | H2O | Br | rt | - | >99 |
| 2 | H2O | Br | 100 | - | >99 |
| 3 | THF | Br | rt | - | >99 |
| 4 | THF | Br | 65 | - | >99 |
| 5 | THF/H2O | Br | rt | - | >99 |
| 6 | THF/H2O | Br | 65 | - | >99 |
| 7 | TBAF | Br | 95 | - | >99 |
| 8 | [bmim]Cl | Br | 95 | 70 | 30 |
| 9 | [bmim]Cl | Cl | 95 | 60 | 40 |
| 10 | [bmim]Cl | I | 95 | 3 | 97 |
| 11 | [bmim]Br | Br | 95 | >99 | - |
| 12 | [bmim]Br | Cl | 95 | >99 | - |
| 13 | [bmim]Br | I | 95 | >99 | - |
| 14 | [(d3)-bmim]Br | Br | 95 | >99 c | - |
| 15 | [bmim]BF4 | Br | 95 | - | >99 |
| 16 | [bmim]PF6 | Br | 95 | - | >99 |
| 17 | [bmpy]F3CSO3 | Br | 95 | - | >99 |
a In (1 equiv.), in IL (2 equiv.), 14 h; b Values of 1 and 2 were determined by GC-MS; c2H-benzene was obtained.
