Table 3.
Dehalogenation of haloheteroaromatics compounds using indium metal in IL a.
| Entry | Substrate | Product | Yield b (%) | Recovery b (%) | |
|---|---|---|---|---|---|
| 1 |
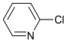
|
14 |
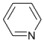
|
- | >99 |
| 2 |
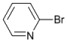
|
14 |

|
>99 | - |
| 3 |
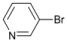
|
14 |

|
60 | 40 |
| 4 |
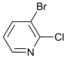
|
15 c 16 d |
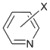
|
20 c 80 d |
- |
| 5 |
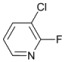
|
17 |
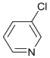
|
- | >99 |
| 6 |
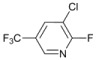
|
18 e 19 f |
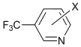
|
60 e30 f | 10 |
| 7 |
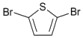
|
20 |
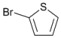
|
90 | 10 |
| 8 |
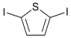
|
21 |
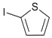
|
>99 | - |
a In (1 equiv), in IL (2 equiv), 95 °C, 14 h; b Values of substrate and product were determined by GC-MS; c 3-bromopyridine; d 2-chloropyridine; e 2-fluoro-5-(trifluoro-methyl)pyridine; f 3-chloro-5-(trifluoromethyl)pyridine.
