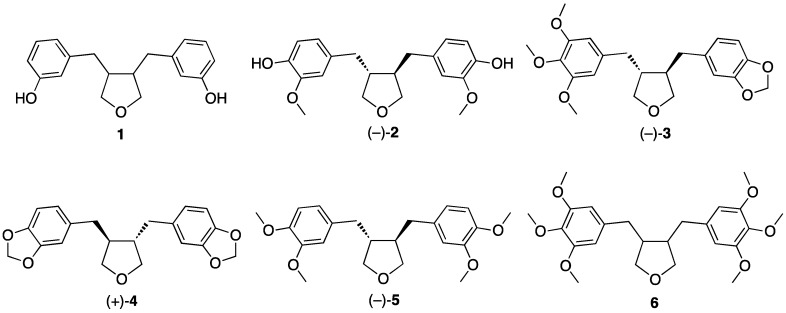
Figure 1.
Naturally occurring 3,4-dibenzyltetrahydrofuran lignans (IUPAC names in parentheses). Mammalian lignan enterofuran (1, 3,3′-dihydroxy-9,9′-epoxylignane), and plant lignans anhydrosecoisolariciresinol aka shonanin (2, 4,4′-dihydroxy-3,3′-dimethoxy-9,9′-epoxylignane) [14,18,19], burseran (3, 3,4,5-trimethoxy-3′,4′-methylenedioxy-9,9′-epoxylignane) [12], dehydroxycubebin (4, (3,4),(3′,4′)-dimethylenedioxy-9,9′-epoxy-lignane) [20], brassilignan (5, 3,3′,4,4′-tetramethoxy-9,9′-epoxylignane) [21], and 6 (3,3′,4,4′,5,5′-hexamethoxy-9,9′-epoxylignane) [22] (there is some confusion however concerning the structure of compound 6: Fuzzatti et al. have measured a positive value of the optical rotation for the isolated trans-3,4-dibenzyltetrahydrofuran lignan, but have drawn the structure as that of the (8R,8′R)-enantiomer [22], which in all the other reported cases is observed to have a negative value).