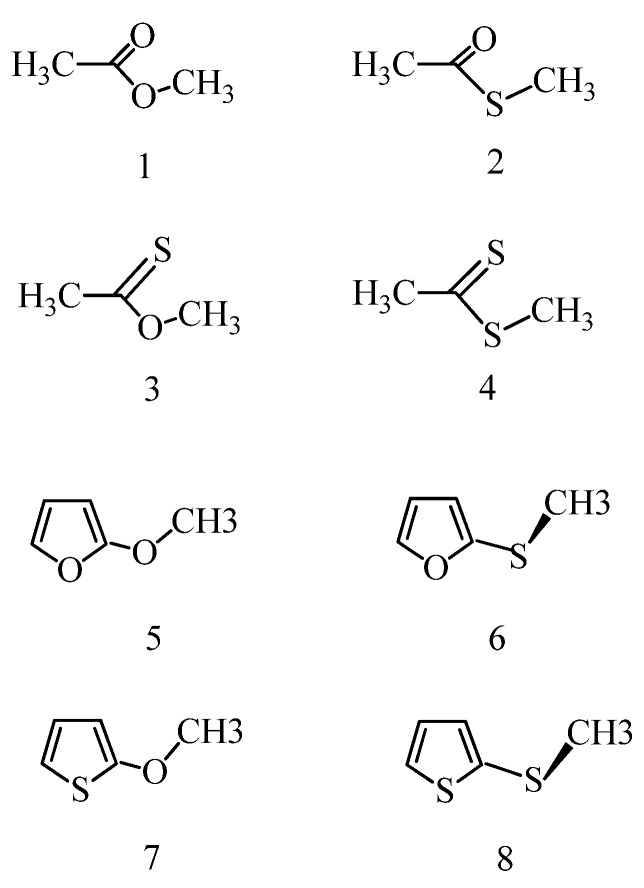
Figure 1.
Compound structures: methyl acetate (1); S-methyl thioacetate (2); O-methyl thioacetate (O-methyl ethanethioate, 3); dithioacetic acid methyl ester (4); 2-methoxyfuran (5); 2-methylthiofuran (6); 2-methoxythiophene (7); 2-methylthiothiophene (8). The indicated conformation of the esters 1–4 is the most stable cis form. For 2-methoxyfuran (5) and 2-methoxythiophene (7) the most stable form is trans, and for 2-methylthiofuran (6) and 2-methylthiothiophene (8) the most stable form is gauche.