Table 1.
Antileishmanial activity and cytotoxicity (compounds 1–13).
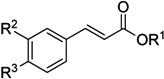
| IC50 (μM) * | |||||||
|---|---|---|---|---|---|---|---|
| Compd. | R1 | R2 | R3 | L. major promastigotes | L. donovani promastigotes | J774.1 | |
| 1 | 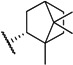 |
-OH | -OH | 48.8 | 27.3 | 8.3 | |
| 2 | -OH | -OCH3 | 64.4 | 41.3 | 48.7 | ||
| 3 | -H | -Cl | 71.2 | >100 | 49.5 | ||
| 4 | -H | -Br | >100 | >100 | 54.6 | ||
| 5 | -H | -N(CH3)2 | >100 | >100 | >100 | ||
| 6 | -H | 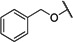 |
>100 | >100 | >100 | ||
| 7 | 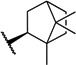 |
-OH | -OH | 45.8 | 34.8 | 8.8 | |
| 8 | -OH | -OCH3 | 60.6 | 74.5 | 44.3 | ||
| 9 | -H | -NO2 | >100 | >100 | >100 | ||
| 10 | 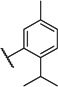 |
-OH | -OH | 57.6 | 42.1 | 9.5 | |
| 11 | -OH | -OCH3 | 59.8 | 79.6 | 45.6 | ||
| 12 | 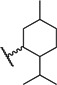 |
-OH | -OH | 59.5 | 79.4 | 1.95 | |
| 13 | -OH | -OCH3 | 54.2 | >100 | 44.6 | ||
* Positive control: pentamidine 82 μM (L. major), 38.6 μM (J774.1); miltefosine: 36.2 μM (L. major), 56.5 μM (J774.1); amphotericin B 0.4 μM (L. donovani).
