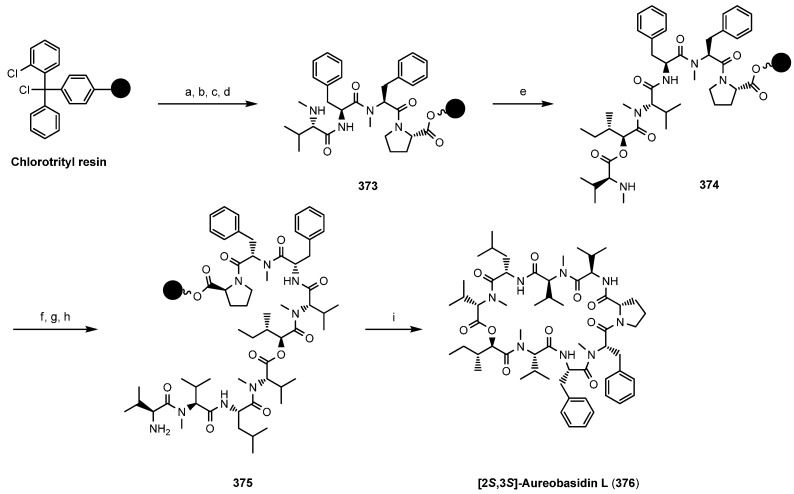
Scheme 21.
Total synthesis of aureobasidin derivative 376 on solid-phase.
Reagents and conditions: (a) 1. Fmoc-l-Pro, CH2Cl2, DIPEA; 2. MeOH; 3. piperidine in DMF; (b) 1. Fmoc-l-N-MePhe, HBTU/HOBt, HATU/HOAt, or BTC (triphosgene)/collidine, DIPEA, CH2Cl2/DMF; 2. piperidine in DMF; (c) 1. Fmoc-l-Phe, HATU/HOAt, DIPEA, CH2Cl2/DMF; 2. piperidine in DMF; (d) Fmoc-l-N-MeVal, HBTU/HOBt or HATU/HOAt, DIPEA, CH2Cl2/DMF; 2. piperidine in DMF; (e) 1. Fmoc-l-N-MeVal-l-Hmp-OH, BTC/collidine, DIPEA, THF; 2. 20% piperidine in DMF (two cycles) or piperidine/DBU/DMF (two cycles); (f) 1. Fmoc-l-Leu, BTC/collidine, DIPEA, THF then HATU/HOAt, DIPEA, CH2Cl2/DMF; 2. 20% piperidine in DMF (two cycles) or piperidine/DBU/DMF, 20 min; (g) 1. Fmoc-l-N-MeVal, BTC/collidine, DIPEA, THF; 2. piperidine/DBU/DMF, 20 min; (h) 1. Fmoc-l-Val, BTC/collidine, DIPEA, THF then HATU/HOAt, DIPEA, CH2Cl2/DMF; 2. piperidine/DBU/DMF, 20 min; (i) 1. TFA/CH2Cl2, 15 min; 2. HATU, DIPEA, CH2Cl2.