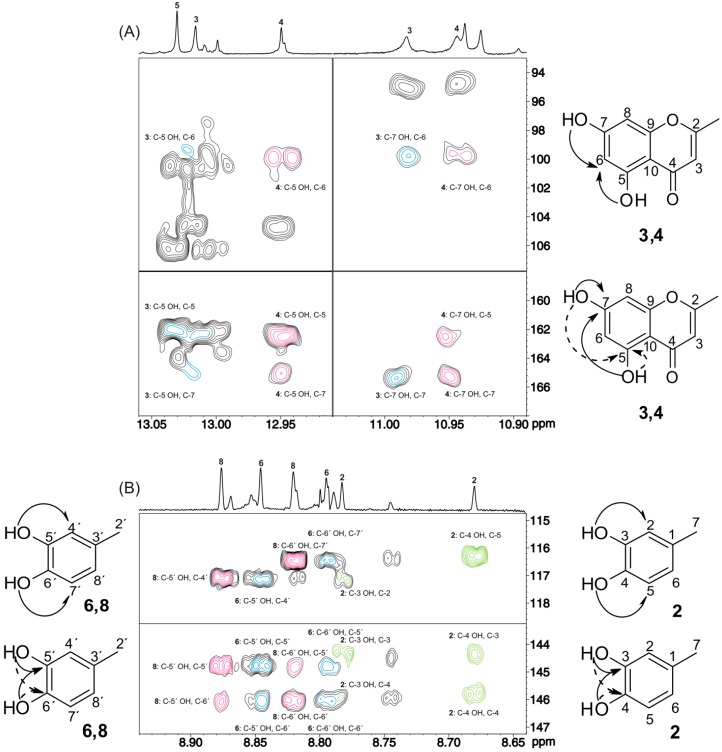
Figure 23.
500 MHz 2D 1H-13C HMBC-NMR spectrum of 10 mg of an olive leaf methanol extract in 0.6 mL of DMSO-d6 with a mass ratio of [picric acid]/[extract] of 49.3 × 10−3 (T = 288 K, number of scans = 88, experimental time = 11 h and 34 min). The experiment was optimized for nJ(1H, 13C) values of 6 to 8 Hz. (A) The common cross-peaks of the C-5 and C-7 hydroxyl protons to carbons C-6, C-5, and C-7 of luteolin-4′-O-β-d-glucopyranoside (4) and the common cross-peaks of the C-5 and C-7 hydroxyl protons to carbons C-6 and C-7 of luteolin (3) are illustrated in red and blue, respectively. (B) The common cross-peaks of the C-5′ and C-6′ hydroxyl protons to carbons C-4′ and C-7′, respectively (upper trace), and the common cross-peaks to carbons C-5′ and C-6′ (lower trace) are illustrated in blue for oleuropein 6-O-β-d-glucopyranoside (6), green for hydroxytyrosol (2), and red for oleuropein (aldehyde form) (8). Reproduced with permission from [16]. Copyright 2011, by the American Chemical Society.