Abstract
Schistosomiasis is a neglected tropical disease that affects hundreds of millions of people worldwide. Since the treatment of this disease currently relies on a single drug, praziquantel, new and safe schistosomicidal agents are urgently required. Nerolidol, a sesquiterpene present in the essential oils of several plants, is found in many foods and was approved by the U.S. Food and Drug Administration. In this study we analysed the in vitro antiparasitic effect of nerolidol on Schistosoma mansoni adult worms. Nerolidol at concentrations of 31.2 and 62.5 μM reduced the worm motor activity and caused the death of all male and female schistosomes, respectively. In addition, confocal laser scanning microscopy revealed morphological alterations on the tegument of worms such as disintegration, sloughing and erosion of the surface, and a correlation between viability and tegumental damage was observed. In conclusion, nerolidol may be a promising lead compound for the development of antischistosomal natural agents.
Keywords: Schistosoma, schistosomiasis, nerolidol, schistosomicial activity, natural product, neglected tropical disease, in vitro studies, confocal laser scanning microscopy
1. Introduction
Schistosomiasis is a neglected tropical disease caused by parasitic flatworms of the genus Schistosoma. It is one of the most prevalent parasitic diseases in tropical and sub-tropical areas of the world, and is one of the leading causes of morbidity and mortality in endemic countries. As reviewed elsewhere, it is estimated that more than 200 million people have been infected and approximately 800 million, mostly children, live at risk of infection [1]. Schistosomiasis leads to a chronic, often debilitating, disease that impairs growth, development and productivity in infected individuals, and is strongly linked to extreme poverty. The disease results in about 300,000 deaths annually in sub-Saharan Africa alone and the disease burden, measured in disability-adjusted life-years, is estimated to exceed 70 million [2,3]. At least three species infect humans, namely Schistosoma mansoni, S. japonicum and S. haematobium. The major aetiological agent of human schistosomiasis is S. mansoni and the adult worms colonize the veins of the portal system and can live there for many years [4].
The treatment and control of schistosomiasis relies on a single drug, praziquantel, which has been administered to millions of people yearly since it was developed in the 1970s. However, the occurrence of praziquantel failures in the field or in the laboratory has been described [5,6]. A reliable alternative to praziquantel does not currently exist and the older drug oxamniquine is no longer manufactured. Thus, the resulting dependence on a single drug for the treatment of schistosomiasis is not sustainable, and for this reason, new and safe schistosomicidal agents are urgently required [7,8].
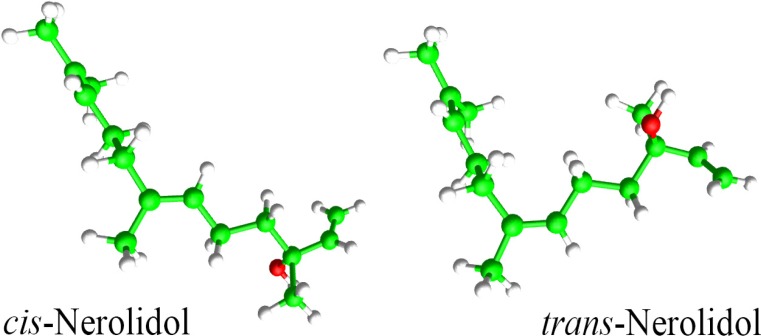
Natural products have been the basis of treatment for many human diseases [9,10]. Essential oils are highly enriched in compounds termed terpenoids that possess several biological properties such as antiparasitic activity [9,11,12]. Nerolidol (3,7,11-trimethyl-1,6,10-dodecatrien-3-ol), also known as peruviol, is an aliphatic sesquiterpene alcohol present in essential oils of several plants (Figure 1). It is frequently used in cosmetics (e.g., shampoos and perfumes) and in non-cosmetic products (e.g., detergents and cleansers) [13,14]. In medicinal fields, nerolidol has shown antioxidant [15], antinociceptive [16] and antiulcer [17] activities. Nerolidol is active against bacteria and fungi [18,19,20]. With respect to the antiparasitic effect of nerolidol, it has shown antileishmanial [21], antitrypanosomal [22] and antimalarial [23] activities as well as inhibitory effect on the growth of Babesia parasites [24]. On the other hand, there is little data related to the anthelmintic activity of nerolidol.
Figure 1.
Chemical structure of nerolidol: cis-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol and trans-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol.
Various plant essential oils have also been investigated for use as antiparasitic agents. In this regard, the search for antischistosomal compounds from natural sources has intensified [25,26]. In the present study, we describe the antischistosomal activity of the terpene nerolidol and show its effect in the tegument of adult parasites by confocal laser scanning microscopy studies. Nerolidol was selected for this study based on the fact that this compound has well-characterised mechanisms of toxicity and is easily available and cost-effective. Additionally, nerolidol is naturally present in many foods we eat, and is approved by the U.S. Food and Drug Administration as Generally Recognized as Safe (GRAS) and was included by the Council of Europe in the list of substances granted [13,14].
2. Results and Discussion
Medicinal plants have been used for hundreds of years as therapeutics worldwide and the interest in natural products as new sources of antischistosomal drugs is rising. In this study, 49-day-old adult S. mansoni were cultured in RPMI 1640 medium in the presence of nerolidol, a sequisterpene present in essential oils of several plants.
2.1. Nerolidol Affected the Viability of Schistosomes
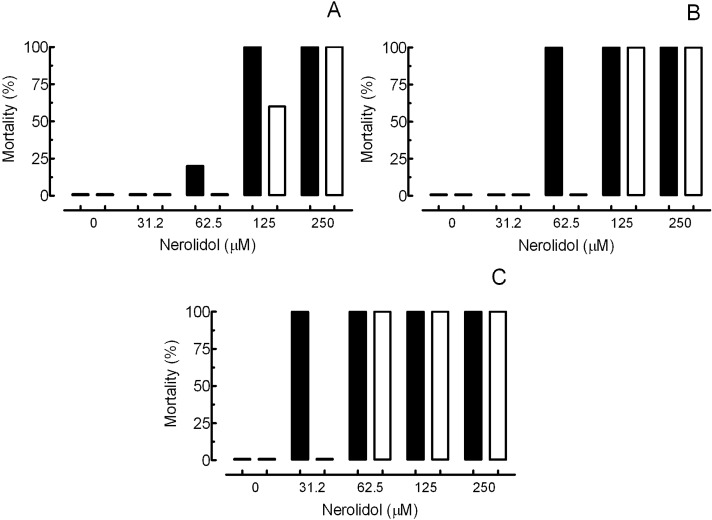
The results of the in vitro studies with schistosomes exposed to nerolidol at concentrations of 15.6, 31.2, 62.5, 125 and 250 μM and control groups are summarised in Figure 2 and Table 1. In the negative control group (RPMI 1640 medium containing 0.5% DMSO), schistosomes showed normal motor activity and had no observed mortality. In contrast, 3 µM praziquantel resulted in complete loss of motor activity and caused the death of all parasites. These observations in the negative and positive control groups are similar to that described in the literature [27,28,29].
Figure 2.
In vitro effect of nerolidol on the survival of Schistosoma mansoni after 24 h (A), 48 h (B) and 120 h (C) of treatment. Pairs of adult worms, males (closed bars) and females (open bars), were incubated in 24-well culture plates containing RPMI 1640 medium and treated with nerolidol at different concentrations. Mortality data are presented from ten worm couples and values correspond to the sum of the adult schistosomes obtained from three separate experiments performed in triplicate (n = 2) and quadruplicate (n = 1).
Table 1.
In vitro effects of nerolidol against adult Schistosoma mansoni.
| Group | Period of incubation (h) | Separated worms (%) a | Dead worms (%) a | Motor activity reduction (%) a | ||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | |||
| Control b | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5% DMSO | 48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 72 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 96 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 120 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PZQ | 24 | 0 | 100 | 100 | 0 | 0 | 100 | 100 |
| 3 µM | 48 | 0 | 100 | 100 | 0 | 0 | 100 | 100 |
| 72 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | |
| 96 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | |
| 120 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | |
| Nerolidol | 24 | 100 | 100 | 100 | 0 | 0 | 100 | 100 |
| 250 µM | 48 | 100 | 100 | 100 | 0 | 0 | 100 | 100 |
| 72 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
| 96 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
| 120 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
| Nerolidol | 24 | 100 | 100 | 60 | 0 | 0 | 100 | 100 |
| 125 µM | 48 | 100 | 100 | 100 | 0 | 0 | 100 | 100 |
| 72 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
| 96 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
| 120 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
| Nerolidol | 24 | 100 | 20 | 0 | 0 | 60 | 100 | 0 |
| 62.5 µM | 48 | 100 | 100 | 0 | 0 | 40 | 100 | 60 |
| 72 | 100 | 100 | 0 | 0 | 0 | 100 | 100 | |
| 96 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
| 120 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | |
| Nerolidol | 24 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| 31.2 µM | 48 | 100 | 0 | 0 | 40 | 0 | 0 | 0 |
| 72 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 96 | 100 | 100 | 0 | 0 | 100 | 100 | 0 | |
| 120 | 100 | 100 | 0 | 0 | 100 | 100 | 0 | |
| Nerolidol | 24 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15.6 µM | 48 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| 72 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 96 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 120 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |
a Percentages relative to the 20 worms investigated. Values correspond to the sum of the adult schistosomes obtained from three separate experiments performed in triplicate (n = 2) and quadruplicate (n = 1). Male parasite (M). Female parasite (F); b RPMI 1640 medium.
During incubation with nerolidol (15.6 to 250 μM), all of the adult worm pairs were separated into individual male and female worms. Interestingly, nerolidol at 62.5 μM was lethal to 100% of male adult parasites after 48 or 72 h of exposure in vitro, whereas in this same time period, no mortality was observed in the female worms (Figure 2 and Table 1). However, nerolidol at 250 and 125 μM significantly reduced motor activity in S. mansoni and resulted in death of 100% of male and female parasites on first and second day of incubation, respectively (Table 1). These findings show that the nerolidol has antischistosomal properties as well as indicate that adult male parasites are more susceptible to the nerolidol than female worms. A similar variation in drug susceptibility between male and female schistosomes have also been observed for praziquantel [30] and with other natural products such as volatile organic components of Ageratum conyzoides [31] and ginger rhizomes [32].
2.2. Nerolidol Caused Tegumental Damage in Schistosomes
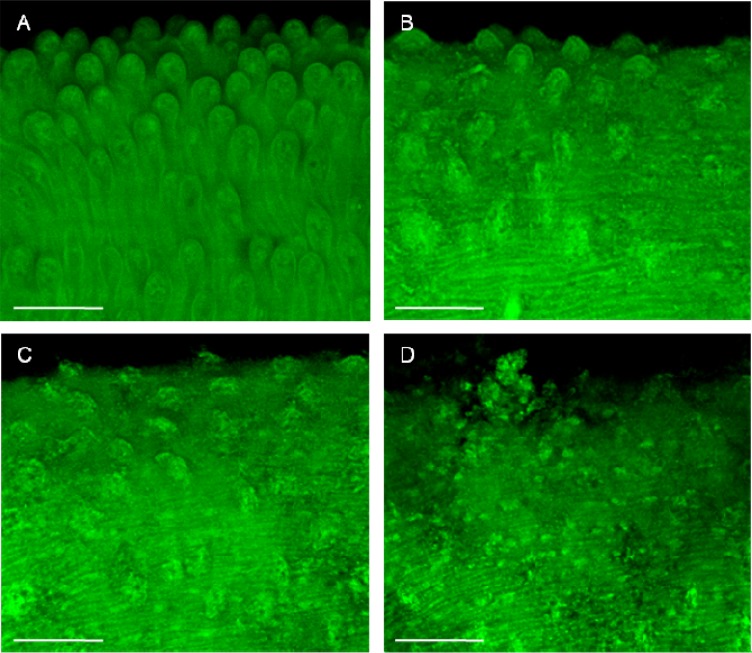
The schistosome worm is covered by a syncytial cytoplasmic layer, the tegument, which is of crucial importance for parasite survival [33]. Thus, the worm tegument is considered an important drug target in schistosomiasis [26]. We used confocal laser scanning microscopy as a tool to evaluate whether the exposure to nerolidol could affect the tegument of S. mansoni adult worms. The morphological features of adult S. mansoni in the control groups were in agreement with previous reports [27,34,35]. As can be observed in Figure 3, no abnormality was noticed in S. mansoni adult worms in the negative control group and, thus, the dorsal tegumental surface of male worms showed intact tubercles (Figure 3A). In contrast, nerolidol at 62.5 to 250 µM caused morphological alterations in the tegument of parasites. For example, slight tegumental damage was observed in the schistosomes treated with nerolidol at 62.5 µM (Figure 3B), whereas extensive tegumental damages were observed at 125 and 250 µM (Figure 3C,D). The principal alterations in S. mansoni male worms exposed to nerolidol were disintegrated tubercles as well as sloughing and erosion of the surface.
Figure 3.
Microscopy investigation of Schistosoma mansoni male worm after in vitro incubation with nerolidol. After 5 days or in the case of death, schistosomes were fixed and monitored using a confocal microscopy. (A) Negative control. (B) 62.5 µM nerolidol. (C) 125 µM nerolidol. (D) 250 µM nerolidol. Scale bars = 50 µm.
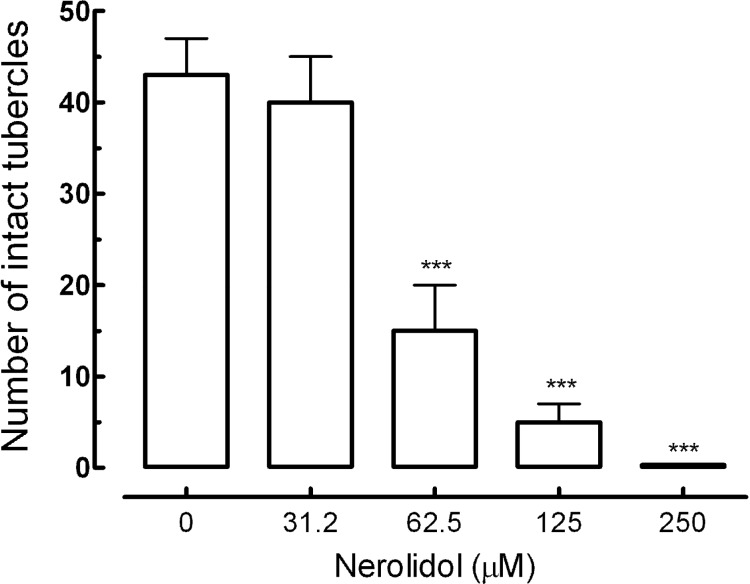
Additionally, we performed a quantitative analysis to observe tegumental damage on S. mansoni male worm. In this case, areas of 20,000 µm2 of the tegument of schistosomes were assessed, and the number of tubercles was counted. Nerolidol caused changes in the tubercles of schistosomes in a concentration-dependent manner. As can be observed in Figure 4, the number of normal tubercles on schistosomes of the control group was 43 (± 4), while in the parasites treated with 62.5 µM of nerolidol, the number was 15 (± 5). In addition, when the concentration of nerolidol was increased to 250 μM, no intact tubercles were seen.
Figure 4.
Morphological changes on the tegument of S. mansoni male worms after treatment with nerolidol. Quantitative analysis, measured in a 20,000 µm2 of area in a dorsal region of male parasite, was performed using three-dimensional images obtained from confocal microscope (see Figure 3). A minimum of three tegument areas of each parasite were assessed. Values are means ± SD (bars) of ten male adult worms. *** p < 0.001 compared with untreated groups.
A recent study reported that nerolidol (10 to 100 μM) did not show any antischistosomal activity against S. mansoni adult worms [36]. However, in our hands, nerolidol exhibited antischistosomal properties at lower concentrations (31.2 and 62.5 µM). It has been demonstrated that different Schistosoma strains exhibit different drug sensitivity patterns [37] and thus we attribute the differences between our results and previous data to the use of different S. mansoni strains. Moreover, the researchers used trans-nerolidol [36], whereas we used a racemic mixture; therefore, it is possible that trans-nerolidol is the far less active isomer. Indeed, the results in the present study showed that the nerolidol has antischistosomal properties as well as revealed that nerolidol induced severe tegumental damage in adult schistosomes. Additionally, quantitative analysis showed that nerolidol caused alterations on the tubercles of male parasites in a concentration-dependent manner.
The mechanism by which nerolidol exerts its in vitro schistosomicidal effect is not clear. However, a correlation between viability and tegumental damage was observed (Table 1, Figure 2, Figure 3 and Figure 4). Due to its inherent lipophilicity, nerolidol will easily cross plasmatic membranes [19] and, consequently, may also interact with intracellular molecules of parasites. Comparable results were obtained by previous works using other antischistosomal natural compounds, such as epiisopiloturine [38], (+)-limonene epoxide [39], dermaseptin [40] and phytol [41].
3. Experimental
3.1. Drugs
Nerolidol (a mixture of cis- and trans-nerolidol) (Figure 1) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and praziquantel was purchased from Merck (São Paulo, SP, Brazil). Stock solutions (8 mM nerolidol and 4 mM praziquantel) were prepared in dimethyl sulfoxide (DMSO, Sigma-Aldrich) and were used for in vitro experiments.
3.2. Parasite
Schistosoma mansoni (BH strain) was used in this study. Schistosomes were obtained from experimentally infected Mesocricetus auratus hamsters as described previously [26,34]. Animals were subcutaneously infected with approximately 150 cercariae following standard procedures of our laboratory [26]. Seven weeks post-infection, adult S. mansoni were removed from the hepatic portal system and mesenteric veins and cultured in RPMI 1640 culture medium supplemented with 200 IU/mL penicillin and 200 µg/mL streptomycin (Invitrogen, São Paulo, SP, Brazil) 10% and foetal bovine serum at 37 °C in an atmosphere of 5% CO2 until use.
3.3. In Vitro Antischistosomal Assay
For the in vitro test with S. mansoni, parasites were incubated in a 24-well culture plate (TPP, St. Louis, MO, USA), placing one coupled worm pair in each well, containing the RPMI 1640 medium at 37 °C in a 5% CO2 atmosphere [27,35]. Nerolidol was used at concentrations of 15.6 to 250 μM (15.6, 31.2, 62.5, 125 and 250 μM) in culture plates with a final volume of 2 mL. The parasites were kept for 120 h and monitored every 24 h using an inverted microscope. The effect of the drug was assessed with emphasis on changes in worm motor activity and alteration in the tegument as previously described [41]. Death was defined as no movement observed for at least 1 to 2 min of examination [26,42]. In addition, worms were prepared for confocal laser scanning microscopy examination whose details are described below. The control worms were assayed in RPMI 1640 medium as a negative control group and 3 μM praziquantel as a positive control group.
3.4. Microscopy Studies
To observe morphological alterations on the tegument of schistosomes after in vitro assays, male worms were monitored using a confocal laser scanning microscope as described elsewhere [26]. The parasites were fixed in a formalin-acetic acid- alcohol solution (FAA) and analysed under a confocal laser scanning microscope (LSM 510 META, Carl Zeiss, Standorf Göttingen, Vertrieb, Germany). Autofluorescence was excited with a 488 nm line from an Argon laser, and emitted light was collected at 505 nm [43].
To assess the damage in the tegument of S. mansoni as a quantitative method, areas (20,000 µm2) of the dorsal surface of male worms were assessed, and the numbers of tubercles were counted using three-dimensional images obtained from confocal microscopy according to standard procedures [26]. The area was calculated using LSM Image Browser software (Zeiss).
4. Conclusions
The results of the present study show that a racemic mixture of E- and Z-nerolidol possesses in vitro antischistosomal activity against Schistosoma mansoni adult worms. This terpene decreased the motor activity and caused the death of worms; additionally, nerolidol was able to cause morphological alterations in the tegument of adult schistosomes. In general, our results are important as there is an urgent need to develop new agents against schistosomiasis. Since nerolidol is generally recognized as safe, this compound may have antiparasitic applications. Further studies are necessary to elucidate mechanisms of action of nerolidol as well as to examine the in vivo effects of this natural compound in S. mansoni-infected animals.
Acknowledgments
We thank Jefferson S. Rodrigues for excellent technical help with S. mansoni life cycle maintenance at the Adolfo Lutz Institute (São Paulo, SP, Brazil). We are indebted to Henrique K. Roffato and Ronaldo Z. Mendonça (Butantan Institute, São Paulo, SP, Brazil) for assistance confocal microscopy (FAPESP, project 00/11624-5). J. Moraes is grateful to the Faculdade de Ciências de Guarulhos (FACIG/UNIESP) for financial support.
Author Contributions
Marcos P.N. Silva and Josué de Moraes conceived and designed the experiments, Marcos P.N. Silva and Josué de Moraes performed the experiments, Marcos P.N. Silva and Josué de Moraes analyzed the data. George L.S. Oliveira, Rusbene B.F. de Carvalho, Damião P. de Sousa, Rivelilson M. Freitas, Pedro L.S. Pinto and Josué de Moraes contributed reagents/materials/analysis tools. Marcos P.N. Silva and Josué de Moraes wrote the paper.
Conflictts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W., Nagelkerke N.J., Habbema J.D., Engels D. Quantification of clinical morbidity associated with schistosome infection in sub Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/S0001-706X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 3.Gray D.J., McManus D.P., Li Y., Williams G.M., Bergquist R. Schistosomiasis elimination: Lessons from the past guide the future. Lancet Infect. Dis. 2010;10:733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- 4.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 5.Fallon P.G., Doenhoff M.J. Drug-resistant schistosomiasis: Resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am. J. Trop Med. Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 6.Ismail M., Metwally A., Farghaly A., Bruce J., Tao L.F. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am. J. Trop. Med. Hyg. 1996;55:214–218. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 7.Botros S., Bennett J. Praziquantel resistance. Expert Opin. Drug Discov. 2007;2:535–540. doi: 10.1517/17460441.2.S1.S35. [DOI] [PubMed] [Google Scholar]
- 8.Caffrey C.R. Chemotherapy of schistosomiasis: Present and future. Curr. Opin. Chem. Biol. 2007;11:433–439. doi: 10.1016/j.cbpa.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Kayser O., Kiderlen A.F., Croft S.L. Natural products as antiparasitic drugs. Parasitol. Res. 2003;2:55–62. doi: 10.1007/s00436-002-0768-3. [DOI] [PubMed] [Google Scholar]
- 10.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;4:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthony J.P., Fyfe L., Smith H. Plant active components - a resource for antiparasitic agents? Trends Parasitol. 2005;10:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Lapczynski A., Bhatia S.P., Letizia C.S., Api A.M. Fragrance material review on nerolidol (isomer unspecified) Food Chem. Toxicol. 2008;46:S247–S250. doi: 10.1016/j.fct.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 14.McGinty D., Letizia C.S., Api A.M. Addendum to Fragrance material review on Nerolidol (isomer unspecified) Food Chem. Toxicol. 2010;48:S43–S45. doi: 10.1016/j.fct.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Nogueira Neto J.D., de Almeida A.A., da Silva Oliveira J., Dos Santos P.S., de Sousa D.P., de Freitas R.M. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem. Res. 2013;38:1861–1870. doi: 10.1007/s11064-013-1092-2. [DOI] [PubMed] [Google Scholar]
- 16.Koudou J., Abena A.A., Ngaissona P., Bessière J.M. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia. 2005;76:700–703. doi: 10.1016/j.fitote.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Klopell F.C., Lemos M., Sousa J.P., Comunello E., Maistro E.L., Bastos J.K., de Andrade S.F. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae) Z Naturforsch. C. 2007;62:537–542. doi: 10.1515/znc-2007-7-812. [DOI] [PubMed] [Google Scholar]
- 18.Brehm-Stecher B.F., Johnson E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob Agents Chemother. 2003;10:3357–3360. doi: 10.1128/AAC.47.10.3357-3360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park M.J., Gwak K.S., Yang I., Kim K.W., Jeung E.B., Chang J.W., Choi I.G. Effect of citral, eugenol, nerolidol and alpha-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia. 2009;80:290–296. doi: 10.1016/j.fitote.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Johann S., Oliveira F.B., Siqueira E.P., Cisalpino P.S., Rosa C.A., Alves T.M., Zani C.L., Cota B.B. Activity of compounds isolated from Baccharis dracunculifolia D.C. (Asteraceae) against Paracoccidioide brasiliensis. Med. Mycol. 2012;8:843–851. doi: 10.3109/13693786.2012.678903. [DOI] [PubMed] [Google Scholar]
- 21.Arruda D.C., D’Alexandri F.L., Katzin A.M., Uliana S.R. Antileishmanial activity of the terpene nerolidol. Antimicrob Agents Chemother. 2005;5:1679–1687. doi: 10.1128/AAC.49.5.1679-1687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoet S., Stévigny C., Hérent M.F., Quetin-Leclercq J. Antitrypanosomal compounds from the leaf essential oil of Strychnos spinosa. Planta Med. 2006;5:480–482. doi: 10.1055/s-2005-916255. [DOI] [PubMed] [Google Scholar]
- 23.Lopes N.P., Kato M.J., Andrade E.H., Maia J.G., Yoshida M., Planchart A.R., Katzin A.M. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiãpi Amazon Indians. J. Ethnopharmacol. 1999;67:313–319. doi: 10.1016/S0378-8741(99)00072-0. [DOI] [PubMed] [Google Scholar]
- 24.AbouLaila M., Sivakumar T., Yokoyama N., Igarashi I. Inhibitory effect of terpene nerolidol on the growth of Babesia parasites. Parasitol. Int. 2010;59:278–282. doi: 10.1016/j.parint.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Allegretti S.M., Oliveira C.N.F., Oliveira R.N., Frezza T.F., Rehder V.L.G. The Use of Brazilian Medicinal Plants to Combat Schistosoma mansoni. In: Rokni M.B., editor. Schistosomiasis. InTech; Rijeka, Croatia: 2012. pp. 27–70. [Google Scholar]
- 26.Moraes J. Antischistosomal Natural Compounds: Present Challenges for New Drug Screens. In: Rodriguez-Morales A.J., editor. Current Topics in Tropical Medicine. InTech; Rijeka, Croatia: 2012. pp. 333–358. [Google Scholar]
- 27.De Moraes J., Carvalho A.A., Nakano E., de Almeida A.A., Marques T.H. Anthelmintic activity of carvacryl acetate against Schistosoma mansoni. Parasitol. Res. 2013;112:603–610. doi: 10.1007/s00436-012-3172-7. [DOI] [PubMed] [Google Scholar]
- 28.Magalhães L.G., Kapadia G.J., da Silva Tonuci L.R., Caixeta S.C., Parreira N.A., Rodrigues V., da Silva Filho A.A. In vitro schistosomicidal effects of some phloroglucinol derivatives from Dryopteris species against Schistosoma mansoni adult worms. Parasitol. Res. 2010;106:395–401. doi: 10.1007/s00436-009-1674-8. [DOI] [PubMed] [Google Scholar]
- 29.De Souza F.F., Júnior C.O.R., Fernandes T.S., da Silveira L.S., Rezende C.A.M., de Almeida M.V., de Paula R.G., Rodrigues V., da Silva Filho A.A., Couri M.R.C. Anthelmintic effects of alkylated diamines and amino alcohols against Schistosoma mansoni. Biomed. Res. Int. 2013;2013:783490. doi: 10.1155/2013/783490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pica-Mattoccia L., Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.De Melo N.I., Magalhaes L.G., de Carvalho C.E., Wakabayashi K.A., de P. Aguiar G., Ramos R.C., Mantovani A.L., Turatti I.C., Rodrigues V., Groppo M., et al. Schistosomicidal activity of the essential oil of Ageratum conyzoides L. (Asteraceae) against adult Schistosoma mansoni worms. Molecules. 2011;16:762–273. doi: 10.3390/molecules16010762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanderson L., Bartlett A., Whitfield P.J. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber offlcinale) extract towards adult schistosomes and their egg production. J. Helminthol. 2002;76:241–247. doi: 10.1079/JOH2002116. [DOI] [PubMed] [Google Scholar]
- 33.Skelly P.J., Alan Wilson R. Making sense of the schistosome surface. Adv. Parasitol. 2006;63:185–284. doi: 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- 34.Moraes J., Nascimento C., Lopes P.O., Nakano E., Yamaguchi L.F. Schistosoma mansoni: In vitro schistosomicidal activity of piplartine. Exp. Parasitol. 2011;127:357–364. doi: 10.1016/j.exppara.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 35.De Moraes J., Keiser J., Ingram K., Nascimento C., Yamaguchi L.F. In vitro synergistic interaction between amide piplartine and antimicrobial peptide dermaseptin against Schistosoma mansoni schistosomula and adult worms. Curr. Med. Chem. 2013;20:301–309. doi: 10.2174/092986713804806694. [DOI] [PubMed] [Google Scholar]
- 36.Parreira N.A., Magalhães L.G., Morais D.R., Caixeta S.C., de Sousa J.P., Bastos J.K., Cunha W.R., Silva M.L., Nanayakkara N.P., Rodrigues V., et al. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia. Chem. Biodivers. 2010;7:993–1001. doi: 10.1002/cbdv.200900292. [DOI] [PubMed] [Google Scholar]
- 37.Frezza T.F., de Oliveira C.N.F., Banin T.M., Rehder V.L.G., Boaventura S., Jr., Allegretti S.M. Tegumentary changes in two different strains of Schistosoma mansoni treated with artemisinin and artesunic acid. Rev. Patol. Trop. 2013;42:309–321. [Google Scholar]
- 38.Veras L.M., Guimarães M.A., Campelo Y.D., Vieira M.M., Nascimento C. Activity of epiisopiloturine against Schistosoma mansoni. Curr. Med. Chem. 2012;19:2051–2058. doi: 10.2174/092986712800167347. [DOI] [PubMed] [Google Scholar]
- 39.Moraes Jd., Almeida A.A., Brito M.R., Marques T.H., Lima T.C. Anthelmintic activity of the natural compound (+)-limonene epoxide against Schistosoma mansoni. Planta Med. 2013;79:253–258. doi: 10.1055/s-0032-1328173. [DOI] [PubMed] [Google Scholar]
- 40.De Moraes J., Nascimento C., Miura L.M., Leite J.R., Nakano E. Evaluation of the in vitro activity of dermaseptin 01, a cationic antimicrobial peptide, against Schistosoma mansoni. Chem. Biodivers. 2011;8:548–558. doi: 10.1002/cbdv.201000163. [DOI] [PubMed] [Google Scholar]
- 41.De Moraes J., de Oliveira R.N., Costa J.P., Junior A.L.G., de Sousa D.P., Freitas R.M., Allegretti S.M., Pinto P.L.S. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2014;8:e2617. doi: 10.1371/journal.pntd.0002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manneck T., Haggenmüller Y., Keiser J. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology. 2010;137:85–98. doi: 10.1017/S0031182009990965. [DOI] [PubMed] [Google Scholar]
- 43.Moraes J., Silva M.P., Ohlweiler F.P., Kawano T. Schistosoma mansoni and other larval trematodes in Biomphalaria tenagophila (Planorbidae) from Guarulhos, São Paulo State, Brazil. Rev. Inst. Med. Trop. São Paulo. 2009;51:77–82. doi: 10.1590/s0036-46652009000200004. [DOI] [PubMed] [Google Scholar]