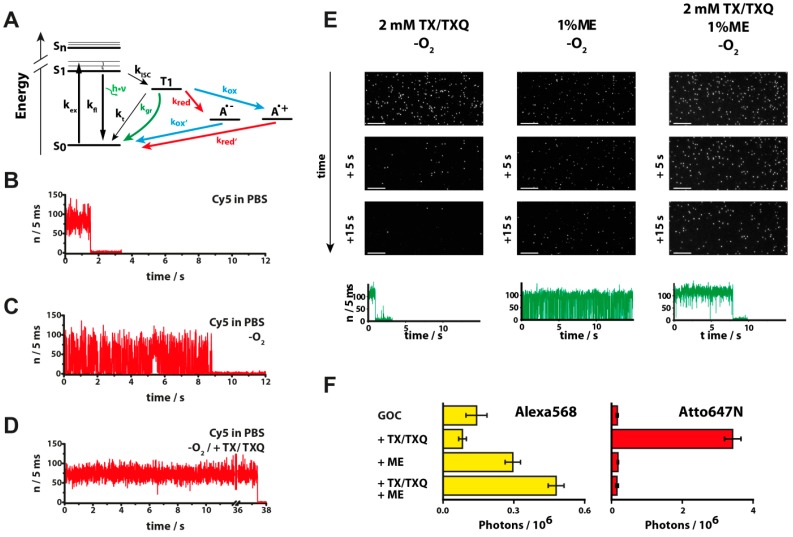
Figure 2.
(A) Jablonski diagram: once an organic dye absorbs a photon it is excited from the ground state S0 to the first excited singlet state S1 (kex) and can return to S0 by emission of a photon (kfl, fluorescence). The dye can undergo numerous excitation-emission cycles before it enters a non-fluorescent triplet state T1 via intersystem crossing (kISC). Once in the triplet state, the dye can either return directly to S0 (kt) via different, competing pathways or can be destroyed by singlet oxygen (photobleaching). The reduction of the dye in its triplet state to a non-fluorescent radical anion A− (kred, red), which can subsequently be oxidized (kox, blue) promotes the depopulation of T1 to S0. The depopulation of the triplet state can also start with the oxidation reaction followed by the reduction step; (B–D) Exemplary photophysical behavior of organic dyes. Fluorescence transients of Cy5-labeled dsDNA in aqueous PBS buffer (B). The removal of oxygen leads to reduced triplet quenching and increased blinking. (C) Addition of 2 mM Trolox(TX)/Troloxquinone (TXQ) enables stable and prolonged fluorescence over minutes (D). Photostabilization by the “geminate recombination” mechanism [48] (E). A time series of frames showing the photostability of Alexa568 dyes in different buffers (2 mM TX/TXQ, left column; 1% beta-mercaptoethanol (ME; center), and a combination of both (right column)), oxygen was removed in all cases (see text for details). Scale bar: 10 μm. The last row shows transients from a confocal microscope that illustrate the differences in blinking and photobleaching behavior of the dye. (F) Total number of detected photons before photobleaching or long-lived dark states for the dyes Alexa568 and ATTO647N in different buffers (figures in panel A, E and F adapted by permission from Holzmeister et al., Geminate recombination as a photoprotection mechanism for fluorescent dyes, Angewandte Chemie, 2014).