Table 2.
Michael addition reaction of barbituric acid derivatives 1 to nitroolefin 2 catalyzed by Et2NH in water at room temperature a.
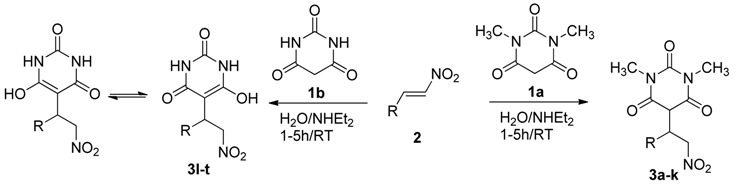
| Entry | 3 | R | Yield (%) b |
|---|---|---|---|
| 1 | 3a | Ph | 99 |
| 2 | 3b | p-CH3Ph | 96 |
| 3 | 3c | p-BrPh | 92 |
| 4 | 3d | p-ClPh | 91 |
| 5 | 3e | 2,4-Cl2Ph | 90 |
| 6 | 3f | 2,6-Cl2Ph | 91 |
| 7 | 3g | p-CH3OPh | 89 |
| 8 | 3h | p-NO2Ph | 88 |
| 9 | 3i | Ferrocene | 93 |
| 10 | 3j | CH3 | 96 |
| 11 | 3k | Thiophene | 95 |
| 12 | 3l | Ph | 97 |
| 13 | 3m | p-CH3Ph | 94 |
| 14 | 3n | p-BrPh | 88 |
| 15 | 3o | p-ClPh | 89 |
| 16 | 3p | 2,4-Cl2Ph | 85 |
| 17 | 3q | 2,6-Cl2Ph | 86 |
| 18 | 3r | p-CH3OPh | 88 |
| 19 | 3s | Ferrocene | 92 |
| 20 | 3t | p-NO2Ph | 87 |
a All reactions were carried out with barbituric acid 1 (1.5 mmol), nitroalkene 2 (1.5 mmol) and amine (1.5 mmol) in water (1.5 mL) for the specified time. b Yield of isolated product.
