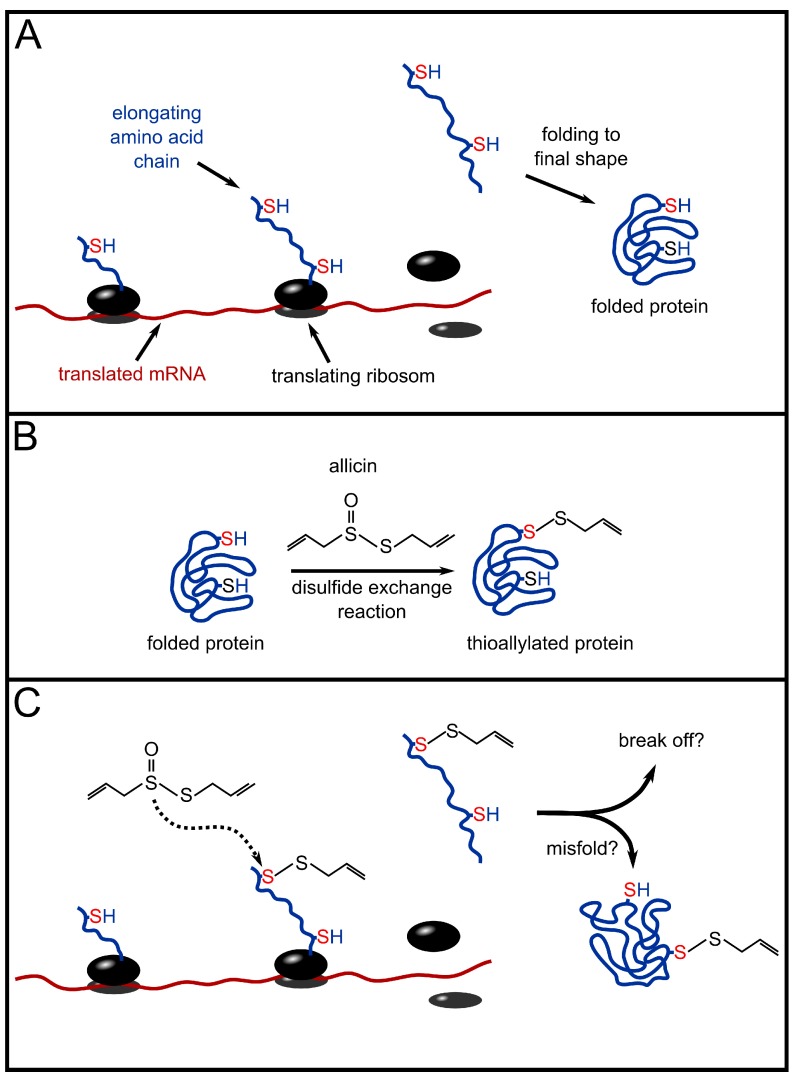
Figure 2.
Possible influence of allicin on proteins and protein synthesis. (A) protein synthesis in unstressed conditions. After translation the protein is folded into its final structure. (B) The cysteine residue that is accessible for attack (indicated in red) reacts with allicin via a disulfide exchange-reaction. The cysteine residue that is sterically blocked (indicated in blue) does not react with allicin. (C) According to Cavallito’s hypothesis, allicin may attack cysteine residues on elongating amino acid chains while the protein is still being synthesized and is not fully developed [48]. In this early stage, cysteine residues that are normally blocked for reactions with allicin (compare B) are now potential targets. Possible results may be an abortion of translation or misfolded proteins with reduced or no function.