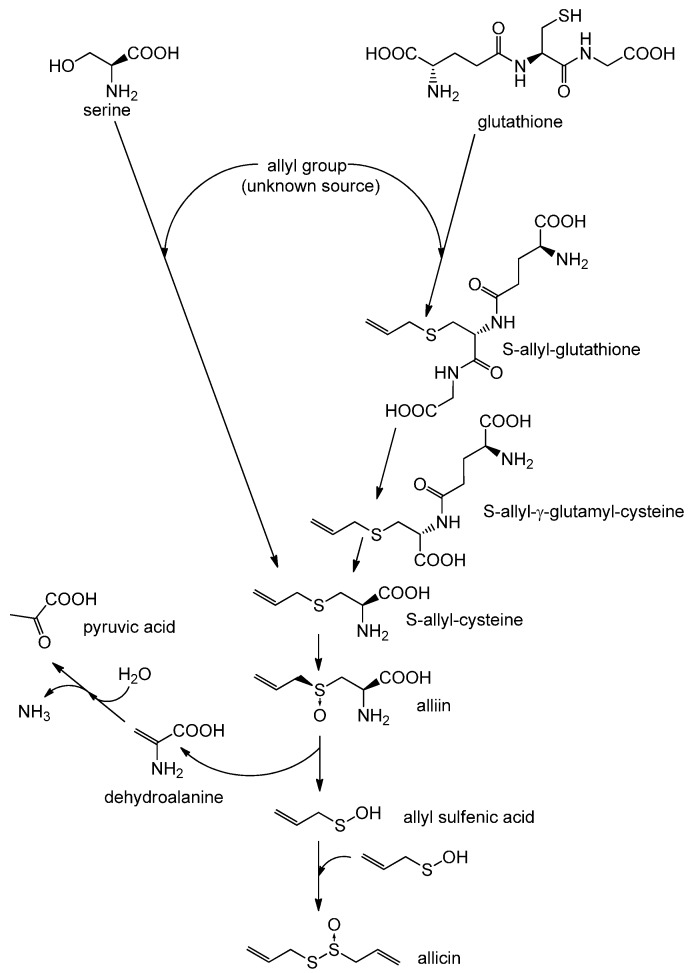
Scheme 1.
Biosynthesis of allicin: Based on Granroth’s work there are two possible biosynthetic pathways leading to S-allyl-cysteine. The detection of 14C-labeled S-allyl-cysteine after feeding plants with 14C-labeled serine and the incorporation of various alkyl mercaptans led Granroth to the conclusion that serine is one possible substrate for S-allyl-cysteine biosynthesis. An alternative pathway leads from glutathione to S-allyl-cysteine. This was confirmed by the detection of S-allyl-glutathione and S-allyl-γ-glutamyl-cysteine. The source of the allyl-group is still unknown. S-allyl-cysteine is oxidised to alliin, which is the “inactive” precursor of allicin. Alliin is enzymatically hydrolysed to produce allyl sulfenic acid which condenses spontaneously to allicin.