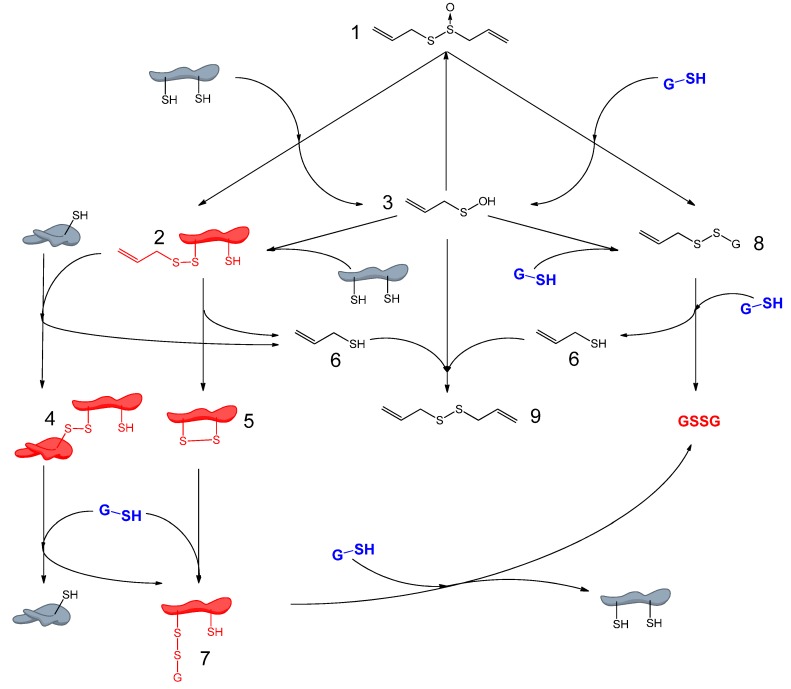
Scheme 3.
Overview of redox chemistry of allicin and cellular thiols: Allicin (1) is able to react with cellular thiols like glutathione (GSH) and cysteine-containing proteins. Reaction with proteins leads to S-allyl-mercapto-proteins (2) and allyl sulfenic acid (3). S-allyl-mercapto-proteins are able to react with other proteins by formation of disulfide bond-stabilised complexes (4) or to form intramolecular disulfide bonds (5). Both reactions lead to elimination of allyl mercaptan (6). Protein disulfide bonds can be reduced by cellular GSH which leads to S-glutathionyl-mercapto-proteins (7). To remove the glutathionyl residues from the proteins another GSH is needed. Allicin also reacts with GSH. This reaction leads to S-allyl-mercapto-glutathione (8) and allyl sulfenic acid (3). S-allyl-mercapto-glutathione can undergo a thiol/disulfide exchange reaction with another GSH to form GSSG and allyl mercaptan (6). Allyl sulfenic acid (3), produced in direct reactions of allicin and thiols is able to react with proteins to form S-allyl-mercapto-proteins (2), with GSH to form S-allyl-mercapto-glutathione (8), with allyl mercaptan (6) to DADS (9) or with another allyl sulfenic acid (3) to form allicin again.