Abstract
The volatile fraction of Ophrys sphegodes Mill. subsp. sphegodes, Ophrys bertolonii subsp. benacensis (Reisigl) O. Danesch, E. Danasch & Ehrend. and Neotinea tridentata (Scop.) R.M. Bateman, Pridgeon & M.W. Case, three orchid species with different pollinator attraction strategies, sampled in vivo and in situ, were evaluated by headspace solid phase microextraction coupled with gas-chromatography and mass spectrometry. The results were compared with the volatile compounds emitted by flowering plant samples picked from the same populations of orchid species. Hydrocarbons, aldehydes, alcohols and terpenes were the major constituents of “in vivo” orchid scents and some distinctive differences in volatile metabolite composition were observed between Ophrys and Neotinea species. Moreover, the odour bouquets of the picked flowering plant samples were different from the in vivo ones and in particular different proportions of the various terpenes and an increase of α-pinene were observed. In conclusion HS/SPME GCMS proved to be a suitable technique for analyzing and distinguishing the volatile fingerprint of different orchid species, sampled in vivo and in situ in a non-disruptive way, with potentially great advantages for ecophysiological studies of rare and endangered species.
Keywords: Orchidaceae, Italy, conservation, floral scent, HS/SPME GCMS
1. Introduction
Investigation into plant and flower scents represents an important field of modern research directed at special biological recognition theories. The scent of a flower is often a complex blend of secondary volatile metabolites, and together with colour and shape is considered to be the main signal attracting pollinators [1,2,3,4], which in turn affect the reproductive performance of plants, their relationships with the environment and, therefore, their conservation (especially for rare and endangered species).
In particular, the variety of shapes, colours and scents present in orchids is seldom found in other plant families. These characteristics contribute to the unique strategies used by orchids to attract pollinators. Many orchid species provide nectar, but other species are deceptive, so they attract pollinators in different ways, the most common strategies being mimicry of nectariferous flowers (e.g., Orchis, Neotinea, Anacamptis), sexual deception (e.g., Ophrys) and provision of shelter (e.g., Serapias) [5].
In deceptive species attractiveness is very important to ensure reproductive success. For instance, orchid species of the genera Ophrys and Neotinea are known to produce complex bouquets of volatiles typically consisting of more than 100 chemical compounds [6,7,8]. The species belonging to these genera are all deceptive, but Ophrys species use a sexual deception strategy, while Neotinea is a food-deceptive genus [5]. The Ophrys species attract and deceive their pollinators through an elaborate sexual mimicry that involves visual cues and volatile semiochemicals that mimic the pheromone of female insects. Food-deceptive orchids mimic rewarding species, some with specific models (e.g., Disa) others with flowers that have the typical characteristics of rewarding plants (i.e., nectar guides, spur, etc.) (e.g., Orchis, Neotinea, Anacamptis) [9]. Moreover, Ophrys species have evolved a high degree of pollinator specificity, while Neotinea species are more generalist.
Despite the importance of volatile compounds for the ecophysiological aspects of plant life, in the literature there are only a few publications on the characterization of orchids’ volatile profiles [10,11]. Flower, as well as whole plant, scents are conventionally analyzed by different methods, usually based on solvent extraction, steam distillation, or supercritical fluid extraction, that use destructive approaches. Moreover, the methods so far applied require that flowers or plants are collected, causing stress and mechanical damage to the plants thus altering their volatile profiles. Following these considerations soft extraction methods are preferred and HS-SPME was chosen to analyze the orchid samples. This technique has been developed for the fractionation of volatile organic compounds in several matrices, but can be also applied for the direct sampling of flower scents [10,12,13]. The method is rapid, solvent-free and inexpensive and reduces sampling stress; moreover it may be easily transferred into the field in order to investigate real in vivo volatile emission.
The aim of this study was to characterize, for the first time, the volatile organic compounds emitted, in vivo, by Ophrys sphegodes Mill. subsp. sphegodes, Ophrys bertolonii subsp. benacensis (Reisigl) P. Delforge and Neotinea tridentata (Scop.) R.M. Bateman, Pridgeon & M.W. Case, three Italian populations of orchid species with different attraction strategies, sampling them in situ, in a non-disruptive way. Moreover, in order to define the most reliable sampling approach for improving knowledge regarding orchids’ ecophysiological aspects, a comparison was made with the volatile profile obtained from flowering plant samples picked from the same orchid populations.
2. Results and Discussion
2.1. Analysis of “in Vivo” VOCs
The volatile compounds emitted by O. sphegodes subsp. sphegodes, O. bertolonii subsp. benacensis and N. tridentata species are listed in Table 1. We identified 67, 93 and 77 compounds, respectively. These VOCs belonged to the major chemical classes, such as hydrocarbons, aldehydes, ketones, alcohols, furans, phenols, free fatty acids and terpenes. As reported in Table 1, the aromatic profiles of the three orchid species showed some distinctive differences.
Table 1.
Identification of volatile organic compounds by HS-SPME-GCMS from “in vivo” orchids.
| Control | O. sphegodes subsp. sphegodes | O. bertolonii subsp. benacensis | N. tridentata | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | RT a | Mean ( n = 3) b | SD c | Mean ( n = 3) d | SD c | Mean ( n = 3) d | SD c | Mean ( n = 3) d | SD c | ||||
| hexane | 1.50 | 0.96 | 0.01 | ND | - | ND | - | 7.144 | 0.03 | ||||
| octane | 2.21 | 0.39 | 0.14 | 0.16 | 0.13 | 0.08 | 0.08 | 0.14 | 0.00 | ||||
| butane | 2.26 | ND | - | ND | - | 0.17 | 0.17 | 1.09 | 0.15 | ||||
| 1-octene | 2.53 | ND | - | ND | - | 0.17 | 0.17 | 0.22 | 0.00 | ||||
| nonane | 3.25 | ND | - | ND | - | 0.22 | 0.22 | 1.36 | 0.32 | ||||
| undecane | 10.25 | 0.73 | 0.65 | 3.566 | 0.00 | ND | - | 0.76 | 0.01 | ||||
| 1-tridecene | 11.85 | 0.07 | 0.00 | ND | - | 0.11 | 0.11 | ND | - | ||||
| dodecane | 14.88 | 0.13 | 0.02 | ND | - | 1.39 | 1.00 | ND | - | ||||
| tridecane | 18.72 | ND | - | 0.11 | 0.00 | ND | - | 0.79 | 0.03 | ||||
| 4-methyl-tetradecane | 20.55 | ND | - | ND | - | 4.694 | 0.53 | 1.25 | 0.11 | ||||
| pentadecane | 21.43 | 0.68 | 1.68 | ND | - | 1.20 | 0.57 | 0.86 | 0.15 | ||||
| nonadecane | 22.47 | 0.73 | 0.00 | 0.71 | 0.00 | 0.21 | 0.19 | ND | - | ||||
| heptadecane | 25.98 | 0.25 | 0.01 | ND | - | 0.73 | 0.57 | ND | - | ||||
| 2-tridecane | 29.86 | 0.59 | 0.00 | ND | - | 0.43 | 0.26 | 0.50 | 0.00 | ||||
| Total | 4.53 | 4.54 | 9.4 | 14.11 | |||||||||
| Aldehydes | |||||||||||||
| pentanal | 4.78 | ND | - | ND | - | 0.15 | 0.16 | 0.11 | 0.01 | ||||
| esanal | 9.02 | 1.48 | 1.97 | 0.42 | 0.34 | 0.64 | 0.44 | 0.33 | 0.03 | ||||
| 3-hexenal | 12.22 | 0.12 | 0.01 | ND | - | 0.50 | 0.36 | 0.16 | 0.10 | ||||
| heptanal | 13.98 | 0.10 | 0.01 | ND | - | 0.65 | 0.81 | 0.15 | 0.01 | ||||
| 3-methyl-2-butenal | 14.51 | 0.48 | 0.27 | ND | - | 0.28 | 0.08 | ND | - | ||||
| 2-hexenal | 15.27 | 1.25 | 0.00 | 0.42 | 0.02 | 0.67 | 0.44 | 1.39 | 0.89 | ||||
| octanal | 18.01 | ND | - | ND | - | 1.83 | 0.49 | 0.29 | 0.00 | ||||
| 2-heptanal | 18.89 | ND | - | ND | - | 0.34 | 0.31 | ND | - | ||||
| nonanal | 21.02 | 0.72 | 1.60 | 1.68 | 0.79 | 3.006 | 0.93 | 7.922 | 1.11 | ||||
| 2-octenal | 21.74 | ND | - | ND | - | 0.16 | 1.90 | ND | - | ||||
| decanal | 23.63 | 0.72 | 0.64 | 0.86 | 0.02 | 3.485 | 0.07 | 7.423 | 0.78 | ||||
| 2-nonanal | 24.13 | ND | - | 1.08 | 0.72 | 0.58 | 0.41 | ND | - | ||||
| undecanal | 25.86 | 0.05 | 0.30 | ND | - | 0.29 | 0.67 | 0.64 | 0.03 | ||||
| benzene acetaldehyde | 26.43 | ND | - | ND | - | 0.05 | 0.04 | 0.06 | 0.01 | ||||
| dodecanal | 28.00 | 0.40 | 1.23 | 0.46 | 0.30 | 4.903 | 0.79 | ND | - | ||||
| 2-ethylbenzaldehyde | 28.33 | 0.26 | 0.21 | ND | - | 0.11 | 0.00 | ND | - | ||||
| Total | 5.59 | 4.92 | 17.63 | 18.47 | |||||||||
| Esters | |||||||||||||
| ethyl acetate | 3.01 | 0.96 | 0.04 | 0.09 | 0.00 | ND | - | 0.69 | 0.01 | ||||
| 2-methylbutanoic acid methyl ester | 5.85 | ND | - | ND | - | ND | - | 12.362 | 0.21 | ||||
| 3-hexen-1-ol-acetate | 19.00 | 0.48 | 0.12 | 1.64 | 0.24 | 1.69 | 0.21 | 0.34 | 0.02 | ||||
| geranyl acetate | 30.64 | 0.33 | 0.00 | ND | - | 0.11 | 0.07 | 0.55 | 0.06 | ||||
| Total | 1.77 | 1.73 | 1.8 | 13.93 | |||||||||
| Ketones | |||||||||||||
| 3-penten-2-one | 5.11 | ND | - | ND | - | 0.54 | 0.54 | 2.04 | 1.12 | ||||
| 2-nonen-4-one | 15.93 | 1.17 | 0.10 | ND | - | 2.32 | 1.88 | 1.84 | 0.67 | ||||
| 2-methyl-6-heptanone | 16.21 | 0.70 | 0.00 | ND | - | 0.12 | 0.08 | ND | - | ||||
| 3-octanone | 16.78 | ND | - | 0.18 | 0.18 | 0.26 | 0.15 | 0.12 | 0.00 | ||||
| acetoin | 17.6 | 2.42 | 1.58 | ND | - | 0.52 | 0.67 | ND | - | ||||
| 2-octanone | 17.86 | 0.32 | 0.09 | 0.22 | 0.24 | 0.81 | 0.49 | 0.20 | 0.03 | ||||
| 1-octen-3-one | 18.39 | 0.37 | 0.00 | ND | - | 0.64 | 0.47 | ND | - | ||||
| pentadecanone | 23.83 | ND | - | 0.33 | 0.00 | 1.26 | 1.78 | 0.06 | 0.00 | ||||
| 3.5-octadien-2-one | 23.92 | ND | - | 0.3 | 0.12 | 9.851 | 1.50 | 0.71 | 0.11 | ||||
| pantoic lactone | 24.39 | ND | - | ND | - | 0.56 | 0.31 | ND | - | ||||
| 2-pentadecanone | 32.8 | ND | - | ND | - | 0.44 | 0.62 | 0.25 | 0.15 | ||||
| Total | 4.97 | 1.03 | 17.3 | 5.22 | |||||||||
| Phenols | |||||||||||||
| anisole | 19.46 | 0.11 | 0.05 | ND | - | 0.39 | 0.36 | 0.16 | 0.01 | ||||
| 4-methylanisole | 22.04 | ND | - | 1.01 | 0.60 | 0.09 | 0.07 | ND | - | ||||
| phenol | 31.61 | ND | - | 9.654 | 0.44 | 0.39 | 0.41 | ND | - | ||||
| 4-methylphenol | 33.29 | 0.07 | 0.00 | 0.77 | 0.04 | 3.53 | 2.77 | ND | - | ||||
| Total | 0.18 | 11.43 | 4.4 | 0.16 | |||||||||
| Alcohols | |||||||||||||
| ethanol | 3.81 | 0.11 | 0.00 | ND | - | 0.11 | 0.16 | ND | - | ||||
| 1-nonanol | 4.03 | 0.21 | 0.00 | ND | - | 0.26 | 0.28 | 1.16 | 0.19 | ||||
| 2-methyl-3-buten-2-ol | 7.42 | 1.32 | 0.05 | ND | - | ND | 0.28 | 1.46 | 0.89 | ||||
| 2-ethyl-1-hexanol | 8.43 | 0.36 | 0.10 | ND | - | ND | - | 0.09 | 0.00 | ||||
| isobutanol | 10.36 | ND | - | ND | - | 0.09 | 0.05 | ND | - | ||||
| 3-pentanol | 11.17 | ND | - | 0.48 | 0.00 | 0.06 | 0.03 | ND | - | ||||
| 1-penten-1-ol | 12.30 | ND | - | 0.02 | 0.00 | ND | - | 0.27 | 0.03 | ||||
| 1-butanol | 12.75 | 0.07 | 0.00 | ND | - | ND | - | 0.09 | 0.00 | ||||
| 1-penten-3-olo | 13.66 | 0.32 | 0.00 | ND | - | 0.36 | 0.31 | 0.36 | 0.11 | ||||
| isoamylalcohol | 15.47 | 0.10 | 0.01 | 0.04 | 0.00 | ND | - | ND | - | ||||
| 1-pentanol | 17.00 | ND | - | ND | - | 0.52 | 0.65 | 0.03 | 0.00 | ||||
| heptanol | 19.20 | 0.22 | 0.17 | 1.64 | 0.28 | ND | - | 0.14 | 0.02 | ||||
| 3-hexen-1-ol | 20.82 | 0.35 | 0.00 | ND | - | 2.19 | 0.44 | ND | - | ||||
| 2-nonanol | 20.89 | 0.72 | 0.60 | 0.90 | 0.87 | 2.19 | 1.93 | ND | - | ||||
| 1-octen-3-ol | 22.55 | 2.94 | 0.89 | ND | - | 0.57 | 0.26 | 0.59 | 0.56 | ||||
| 2-decanol | 26.08 | ND | - | 0.38 | 0.00 | ND | - | ND | - | ||||
| 1-nonanol | 27.04 | ND | - | ND | - | 0.13 | 0.18 | 1.29 | 0.77 | ||||
| 2-undecanol | 28.27 | 0.26 | 0.21 | 0.61 | 0.48 | 0.03 | 0.33 | 0.48 | 0.21 | ||||
| 3-decen-1-ol | 29.59 | ND | - | 1.49 | 0.00 | ND | - | ND | - | ||||
| 1-heptadecanol | 30.46 | ND | - | ND | - | 0.42 | 0.23 | 0.31 | 0.08 | ||||
| 1-dodecanol | 32.23 | ND | - | 0.49 | 0.04 | 0.14 | 0.13 | ND | - | ||||
| isothymol | 34.70 | ND | - | 0.19 | 0.00 | ND | - | ND | - | ||||
| 1.4-benzenediol | 34.86 | 1.08 | 0.77 | 0.11 | 0.00 | 0.69 | 0.18 | ND | - | ||||
| Total | 8.05 | 6.35 | 7.76 | 6.27 | |||||||||
| Furans | |||||||||||||
| 2-methylfuran | 3.19 | ND | - | 0.09 | 0.06 | ND | - | ND | - | ||||
| 4-ethylfuran | 4.21 | 0.21 | 0.00 | ND | - | 0.04 | 0.06 | 0.24 | 0.03 | ||||
| 2-butylfuran | 11.62 | 0.21 | 0.00 | ND | - | ND | - | 0.13 | 0.01 | ||||
| Total | 0.42 | 0.09 | 0.04 | 0.37 | |||||||||
| Terpenes | |||||||||||||
| α-pinene | 6.03 | ND | - | 4.655 | 0.72 | 1.05 | 0.00 | 0.34 | 0.11 | ||||
| thujene | 6.38 | 0.23 | 1.01 | 0.35 | 0.00 | ND | - | 0.44 | 0.03 | ||||
| β-pinene | 9.61 | 0.08 | 0.00 | 2.19 | 0.00 | 0.17 | 0.25 | 4.595 | 1.01 | ||||
| sabinene | 10.55 | ND | - | 1.31 | 0.00 | ND | - | ND | - | ||||
| δ.3.carene | 12.00 | 0.22 | 0.00 | 0.81 | 0.51 | 0.09 | 0.07 | 0.28 | 0.01 | ||||
| β-myrcene | 13.33 | ND | - | 1.22 | 0.30 | ND | - | ND | - | ||||
| d-limonene | 14.69 | 0.04 | 0.27 | 22.131 | 1.68 | 0.15 | 0.31 | ND | - | ||||
| sabinene | 14.74 | 0.04 | 0.02 | ND | - | ND | - | 0.09 | 0.00 | ||||
| terpene | 16.33 | 0.92 | 0.00 | ND | - | ND | - | 0.47 | 0.03 | ||||
| γ-terpinene | 16.45 | ND | - | 1.09 | 0.97 | ND | - | 0.31 | 0.90 | ||||
| o-cymene | 16.91 | ND | - | 1.41 | 0.24 | 0.05 | 0.01 | 0.10 | 0.09 | ||||
| p-cymene | 17.36 | 0.33 | 0.06 | 0.23 | 0.18 | 0.20 | 0.29 | 0.32 | 0.07 | ||||
| α−terpinolene | 17.71 | 0.76 | 1.58 | 0.12 | 0.14 | ND | - | 0.57 | 0.08 | ||||
| α-cyclocitral | 18.56 | ND | - | 0.62 | 0.00 | 1.82 | 2.50 | ND | - | ||||
| sequiterpene | 21.65 | ND | - | 0.42 | 0.16 | ND | - | ND | - | ||||
| isopatchoulane | 22.66 | ND | - | 0.98 | 0.12 | 0.19 | 0.00 | 1.03 | 0.66 | ||||
| trans-linalool oxide | 22.88 | 0.05 | 0.00 | 0.34 | 0.00 | 0.94 | 0.66 | 0.06 | 0.18 | ||||
| cyclosativene | 23.11 | 0.30 | 0.00 | 2.47 | 1.75 | 0.28 | 0.21 | ND | - | ||||
| copaene | 23.40 | ND | - | ND | - | 1.11 | 0.46 | 0.81 | 0.00 | ||||
| isolongifolene | 23.88 | ND | - | 1.46 | 0.12 | ND | - | ND | - | ||||
| 4-thujanol | 24.57 | ND | - | ND | - | 1.09 | 1.14 | ND | - | ||||
| linalool | 24.71 | ND | - | 0.59 | 0.60 | 0.16 | 0.23 | ND | - | ||||
| α-zingibirene | 24.93 | 0.07 | 0.01 | 9.983 | 7.15 | 0.26 | 0.11 | 0.26 | 0.90 | ||||
| sequiterpene | 25.09 | ND | - | 0.90 | 0.31 | ND | - | 0.46 | 0.12 | ||||
| α-bergamottene | 25.49 | 0.22 | 0.00 | ND | - | 0.55 | 0.00 | ND | - | ||||
| caryophillene | 25.60 | ND | - | ND | - | 6.733 | 1.84 | 0.69 | 0.47 | ||||
| 4-terpineol | 25.79 | 1.19 | 0.41 | 2.06 | 0.43 | 1.28 | 1.18 | 0.65 | 0.01 | ||||
| menthol | 26.53 | 0.20 | 2.54 | 2.02 | 1.06 | 0.90 | 1.14 | 0.14 | 0.03 | ||||
| verbenone | 27.66 | 0.24 | 0.00 | 0.55 | 0.45 | 0.60 | 0.47 | 0.25 | 0.01 | ||||
| eucarvone | 27.77 | 0.20 | 0.01 | 1.44 | 1.27 | 0.19 | 0.19 | ND | - | ||||
| naphtalene | 28.91 | 0.14 | 0.00 | 1.12 | 1.04 | 0.20 | 0.09 | 0.50 | 0.04 | ||||
| sesquiphellandrene | 29.09 | ND | - | ND | - | 2.44 | 0.38 | ND | - | ||||
| estragol | 30.07 | 0.28 | 0.35 | ND | - | 0.37 | 0.89 | 0.35 | 0.11 | ||||
| piperitone oxide | 31.39 | ND | - | 0.63 | 0.00 | 0.77 | 0.41 | ND | - | ||||
| ascaridole | 31.78 | ND | - | 0.37 | 0.07 | ND | - | ND | - | ||||
| α-santolene | 33.74 | ND | - | 0.47 | 0.00 | ND | - | ND | - | ||||
| Total | 5.51 | 61.92 | 21.58 | 12.72 | |||||||||
| Free fatty acids | |||||||||||||
| acetic acid | 22.26 | 0.62 | 0.00 | 0.31 | 0.35 | 0.71 | 0.00 | 1.33 | 0.02 | ||||
| formic acid | 23.44 | 0.69 | 0.08 | 11.72 | 0.18 | ND | - | ND | - | ||||
| pivalic acid | 25.21 | ND | - | ND | - | 0.3 | 0.09 | ND | - | ||||
| hexanoic acid | 30.34 | ND | - | 0.32 | 0.21 | 0.42 | 0.00 | 0.15 | 0.00 | ||||
| heptanoic acid | 31.99 | 0.21 | 1.17 | ND | - | 0.45 | 0.29 | 0.35 | 0.11 | ||||
| octanoic acid | 33.08 | ND | - | 0.03 | 0.00 | 0.19 | 1.01 | 0.67 | 0.03 | ||||
| nonanoic acid | 34.14 | 0.14 | 0.49 | 0.39 | 0.00 | 1.33 | 0.74 | 0.25 | 0.12 | ||||
| decanoic acid | 35.41 | ND | - | ND | - | 0.84 | 0.00 | 0.91 | 0.00 | ||||
| benzoic acid | 37.74 | 0.12 | 0.41 | 0.49 | 0.49 | 0.42 | 0.13 | 0.40 | 0.02 | ||||
| Total | 1.77 | 13.25 | 4.66 | 4.05 | |||||||||
| Miscellaneous | |||||||||||||
| acetonitrile | 5.42 | 8.04 | 0.32 | 0.42 | 0.36 | 11.95 | 0.12 | 17.26 | 0.98 | ||||
| dimethyl sulfone | 31.18 | 0.45 | 0.00 | 1.24 | 0.18 | 0.30 | 0.21 | 0.06 | 0.00 | ||||
| Total | 8.49 | 1.65 | 12.25 | 17.32 | |||||||||
a Retention time; b Normalized amount of volatile compounds (percentage) (peak of volatile compound/total peak area of all volatile compounds) of control samples (n = 3); c Standard deviation (±); d Normalized amount of volatile compounds (percentage) (peak of volatile compound/total peak area of all volatile compounds) of O. sphegodes subsp. sphegodes, O. bertolonii subsp. benacensis and N. tridentata living plants (n = 3); ND: not detected; The most representative compounds for all orchid species were labeled with a number.
The difference among VOCs extracted and detected from picked samples respect to “in situ” arisen from two variables. Firstly by sampling “in situ” we observe a general dilution factor of monitored headspace respect the vials (20 mL) because the special equipment is specially designed to promote air circulation to the contained plant during the SPME extraction. The second reason can take place to the cut operations made in order to collect the flowers. Some terpenes are contained into special plant organ, that damaged, are released in a headspaces vial.
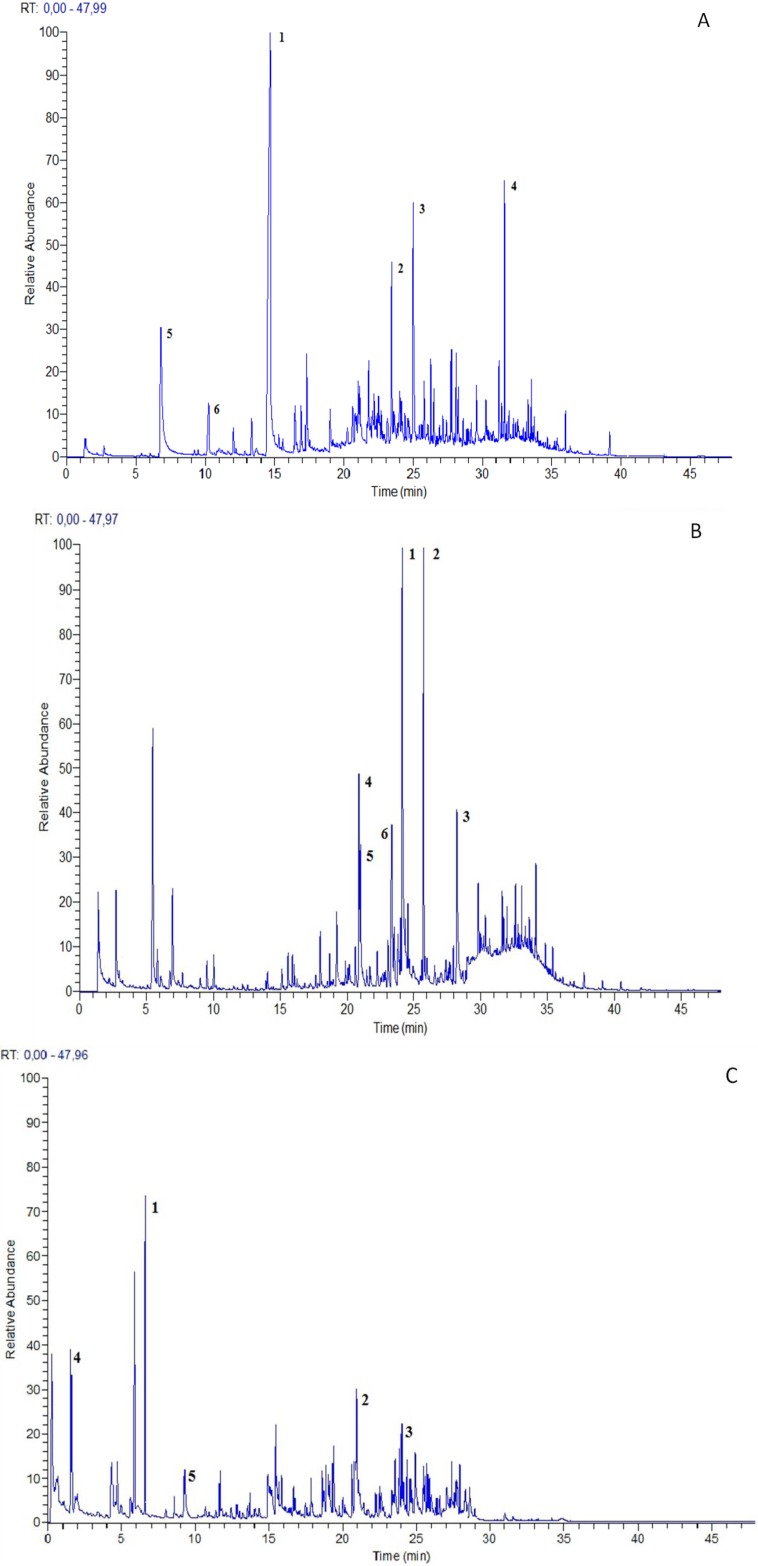
The main components of O. sphegodes subsp. sphegodes bouquet were represented by terpenes, free fatty acids and phenols, followed by alcohols, aldehydes and hydrocarbons in minor amounts. In particular, as reported in Table 1 and Figure 1, the most representative compounds detected were d-limonene (22.13%), formic acid (11.70%), α-zingibirene (9.98%), phenol (9.65%), α−pinene (4.65%) and undecane (3.56%). Among terpenes, in minor amounts, the presence of β−pinene (2.19%) and cyclosativene (2.47%) was revealed. Terpenes, ketones, and aldehydes, were the major constituents of O. bertolonii subsp. benacensis aroma, followed by hydrocarbons and alcohols. In particular 3,5-octadien-2-one (9.85%), caryophillene (6.73%), dodecanal (4.90%), 4-methyltetradecane (4.69%), decanal (3.48%) and nonanal (3%) were the main compounds (Table 1 and Figure 1).
Figure 1.
Volatile fingerprint of O. sphegodes subsp. sphegodes (A), O. bertolonii subsp. benacensis (B) and N. tridentata (C) “in vivo” plant (the most representative compound peaks were labeled with numbers).
O. sphegodes subsp. sphegodes and O. bertolonii subsp. benacensis are sexually deceptive orchids and numerous behavioral tests have shown that the Ophrys-pollinator relationship is highly specific: each Ophrys species is pollinated by males of usually only one or a few pollinator species [14,15,16,17]. In particular the pollinators of O. sphegodes subsp. sphegodes are small bees of the genus Andrena, especially A. nigroaena and A. limata, while O. bertolonii subsp. benacensis O. bertolonii subsp. benacensis is characterized by higher pollinator specificity with the main pollinator being the hymenopterous Chalicodoma parietina [18].
Ophrys flowers usually produce complex bouquets of volatiles, but presumably not all components are important for the attraction of male pollinators [6]. The literature reports that only some hydrocarbons, in particular very long-chain alkanes and alkenes and terpenes act as chemical mimicry of the sex pheromone of the virgin female pollinators. In agreement with our results, other authors confirmed terpenes and hydrocarbons together with alcohols and aldehydes as major constituents of orchid volatile profiles [10,11,19,20] and among these chemical classes a high emission of nonanal and caryophyllene by orchid flowers was previously reported [19,21,22]. In particular, the chemical composition of orchids pollinated by bees (e.g., Ophrys) revealed an abundance of terpenes (mono- and sesquiterpenes) [23].
Not all hydrocarbons, aldehydes and alcohols make an important contribution to the aroma of the plants. Some of them are abundant in cuticular waxes and are said to have the primary function of protecting the plant from dehydration. Other compounds seem to play an important role in plant-herbivore interaction and acting as a solvent for the male-attracting volatiles [24].
The aromatic profile of N. tridentata was characterized by a high concentration of aldehydes, hydrocarbons, esters and terpenes and in particular, as reported in Table 1 and Figure 1, 2-methylbutanoic acid methyl ester (12.36%), nonanal (7.92%), decanal (7.42%), hexane (7.14%) and β-pinene (4.59%).
The genus Neotinea includes food-deceptive species and, in contrast with the Ophrys genus, is thought to attract and deceive mostly naive pollinators by generally mimicking nectariferous plants [5]. N. tridentata is quite generalist in terms of pollinators which are different species of hymenoptera, in particular solitary bees (e.g., Osmia bicolor), Apis mellifera and coleoptera (e.g., Cleridae) [25]. In this genus colour is generally regarded as a primary cue to attract insects to food-deceptive flowers but in this study several odour compounds were found in the floral scents of N. tridentata [5]. As reported in Table 1 most of the VOCs were common to O. sphegodes subsp. sphegodes and O. bertolonii subsp. benacensis flowers but different proportions of the various compounds were observed between Oprhys and Neotinea species. In particular, a minor content of terpenes was identified in N. tridentata bouquet and this difference can be related to the different pollinator attraction strategies. However, there is no confirmation that floral odour is not of importance in pollinator attraction in food-deceptive species, indeed it was observed that in Anacamptis morio, a food-deceptive species, scent emission elicit a response in bee antennae [26].
2.2. Analysis of Picked Flowering Plant VOCs
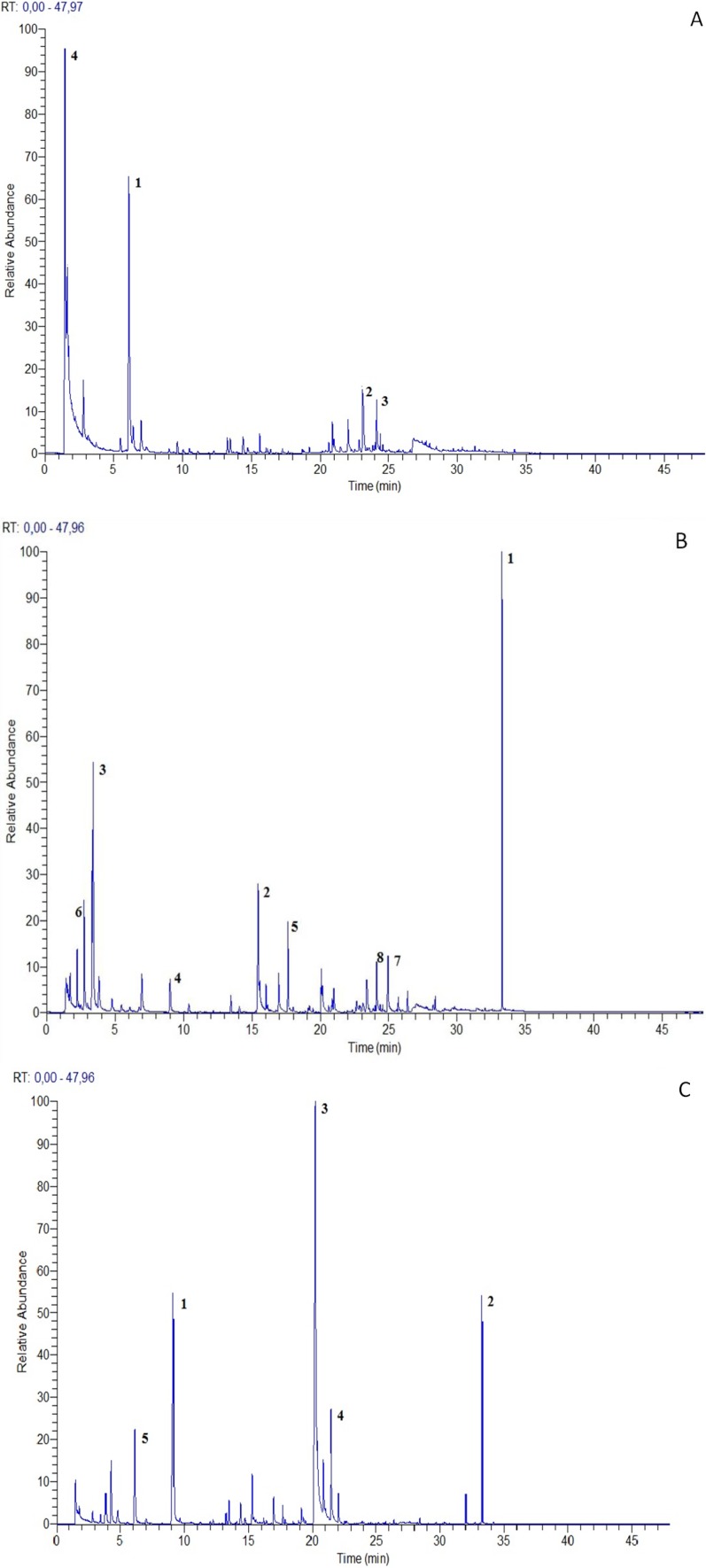
The volatile profile of O. sphegodes subsp. sphegodes, O. bertolonii subsp. benacensis and N. tridentata picked flowering plant samples consists of 56, 53 and 63 compounds, respectively (Table 2). As observed in “in vivo” orchids the aromatic profiles of the three picked flowering plant samples showed some distinctive differences in volatile fingerprint and the most representative compounds of all species were reported in Table 2 and Figure 2.
Table 2.
Identification of volatile organic compounds by HS-SPME-GCMS from picked flowering orchid samples.
| O. sphegodes subsp. sphegodes | O. bertolonii subsp. benacensis | N. tridentata | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | RT a | Mean (n = 3) b | SD d | Mean (n = 3) | SD | Mean (n = 3) | SD | ||||||||||||||||||||||||||||
| Hydrocarbons | |||||||||||||||||||||||||||||||||||
| hexane | 1.50 | 3.874 | 1.00 | 0.41 | 0.10 | 0.20 | 0.00 | ||||||||||||||||||||||||||||
| 2-methylbutadiene | 1.64 | 2.30 | 0.89 | 0.23 | 0.00 | ND | - | ||||||||||||||||||||||||||||
| butane | 2.26 | ND | - | 6.936 | 3.11 | 0.10 | 0.05 | ||||||||||||||||||||||||||||
| nonane | 3.25 | ND | - | 10.873 | 1.31 | 0.06 | 0.01 | ||||||||||||||||||||||||||||
| 1-tridecene | 11.85 | ND | - | ND | - | 0.24 | 0.00 | ||||||||||||||||||||||||||||
| tridecane | 18.71 | 0.33 | 0.02 | ND | - | 0.15 | 0.00 | ||||||||||||||||||||||||||||
| dodecane | 18.79 | 0.17 | 0.01 | ND | - | ND | - | ||||||||||||||||||||||||||||
| pentadecane | 21.45 | 0.26 | 0.03 | ND | - | 7.524 | 1.60 | ||||||||||||||||||||||||||||
| nonadecane | 22.47 | 0.23 | 0.00 | 0.33 | 0.07 | 0.37 | 0.34 | ||||||||||||||||||||||||||||
| heptadecane | 25.98 | 0.13 | 0.02 | 0.17 | 0.00 | 0.06 | 0.00 | ||||||||||||||||||||||||||||
| Total | 7.56 | 18.95 | 11.09 | ||||||||||||||||||||||||||||||||
| Aldehydes | |||||||||||||||||||||||||||||||||||
| pentanal | 4.78 | ND | - | 1.30 | 0.24 | 1.87 | 1.01 | ||||||||||||||||||||||||||||
| esanal | 9.02 | 0.48 | 0.04 | 8.364 | 1.80 | 17.951 | 2.75 | ||||||||||||||||||||||||||||
| 3-hexenal | 12.22 | 0.28 | 0.02 | ND | - | 0.31 | 0.21 | ||||||||||||||||||||||||||||
| heptanal | 13.94 | 0.12 | 0.01 | 0.06 | 0.02 | 0.89 | 0.00 | ||||||||||||||||||||||||||||
| 2-hexenal | 15.27 | 0.04 | 0.00 | 0.51 | 0.00 | 2.43 | 0.67 | ||||||||||||||||||||||||||||
| octanal | 18.01 | 0.08 | 0.01 | 0.32 | 0.01 | ND | - | ||||||||||||||||||||||||||||
| 2-heptanal | 18.87 | ND | - | 0.15 | 0.05 | 0.25 | 0.01 | ||||||||||||||||||||||||||||
| nonanal | 21.00 | 0.40 | 0.03 | 2.31 | 0.69 | ND | - | ||||||||||||||||||||||||||||
| 2-octenal | 21.74 | ND | - | ND | - | ND | - | ||||||||||||||||||||||||||||
| decanal | 23.60 | ND | - | 0.15 | 0.03 | ND | - | ||||||||||||||||||||||||||||
| 2-nonanal | 24.13 | 5.393 | 0.98 | 2.88 | 1.59 | ND | - | ||||||||||||||||||||||||||||
| undecanal | 25.86 | 0.16 | 0.08 | 0.28 | 0.00 | ND | - | ||||||||||||||||||||||||||||
| benzene acetaldehyde | 26.42 | 0.08 | 0.02 | 2.37 | 1.06 | 0.35 | 0.07 | ||||||||||||||||||||||||||||
| dodecanal | 27.98 | 0.37 | 0.11 | ND | - | ND | - | ||||||||||||||||||||||||||||
| 2-ethylbenzaldehyde | 28.33 | ND | - | 0.09 | 0.02 | ND | - | ||||||||||||||||||||||||||||
| Total | 7.41 | 18.79 | 24.04 | ||||||||||||||||||||||||||||||||
| Esters | |||||||||||||||||||||||||||||||||||
| ethyl acetate | 3.04 | ND | - | ND | - | 0.13 | 0.04 | ||||||||||||||||||||||||||||
| 3-hexen-1-ol-acetate | 18.99 | ND | - | ND | - | 0.09 | 0.02 | ||||||||||||||||||||||||||||
| dodecanoic acid. methyl ester | 29.70 | ND | - | ND | - | ND | - | ||||||||||||||||||||||||||||
| geranyl acetate | 30.64 | 0.08 | 0.02 | 0.05 | 0.01 | ND | - | ||||||||||||||||||||||||||||
| Total | 0.08 | 0.05 | 0.21 | ||||||||||||||||||||||||||||||||
| Ketones | |||||||||||||||||||||||||||||||||||
| 2-heptanone | 13.51 | 1.75 | 0.56 | 1.90 | 0.24 | 3.45 | 1.83 | ||||||||||||||||||||||||||||
| 2-nonen-4-one | 15.90 | ND | - | ND | - | 0.12 | 0.00 | ||||||||||||||||||||||||||||
| 2-methyl-6-heptanone | 16.21 | 0.44 | 0.33 | 0.31 | 0.03 | 0.42 | 0.02 | ||||||||||||||||||||||||||||
| 3-octanone | 16.77 | ND | - | ND | - | 0.11 | 0.00 | ||||||||||||||||||||||||||||
| acetoin | 17.60 | 0.19 | 0.01 | 7.125 | 0.13 | 6.81 | 1.87 | ||||||||||||||||||||||||||||
| 2-octanone | 17.87 | ND | - | ND | - | 1.13 | 1.18 | ||||||||||||||||||||||||||||
| pantoic lactone | 24.39 | 0.95 | 0.03 | 0.20 | 0.10 | 0.07 | 0.02 | ||||||||||||||||||||||||||||
| Total | 3.33 | 9.52 | 12.1 | ||||||||||||||||||||||||||||||||
| Phenols | |||||||||||||||||||||||||||||||||||
| anisole | 19.5 | ND | - | 0.31 | 0.15 | 0.17 | 0.01 | ||||||||||||||||||||||||||||
| 4-methyl anisole | 22.01 | 3.53 | 1.01 | 0.15 | 0.01 | 2.40 | 0.18 | ||||||||||||||||||||||||||||
| phenol | 31.58 | 0.15 | 0.02 | ND | - | ND | - | ||||||||||||||||||||||||||||
| 4-methylphenol | 33.27 | 0.23 | 0.01 | 13.251 | 1.30 | 11.282 | 1.23 | ||||||||||||||||||||||||||||
| Total | 3.91 | 13.7 | 13.84 | ||||||||||||||||||||||||||||||||
| Alcohols | |||||||||||||||||||||||||||||||||||
| ethanol | 3.82 | ND | - | 3.11 | 0.61 | 3.28 | 0.56 | ||||||||||||||||||||||||||||
| 2-methyl-3-buten-2-ol | 7.38 | 1.20 | 0.09 | ND | - | ND | - | ||||||||||||||||||||||||||||
| isobutanol | 10.39 | ND | - | 0.64 | 0.31 | 0.12 | 0.00 | ||||||||||||||||||||||||||||
| 1-butanol | 12.71 | ND | - | 0.05 | 0.00 | 0.09 | 0.03 | ||||||||||||||||||||||||||||
| 1-penten-3-ol | 13.00 | ND | - | ND | - | 6.37 | 0.11 | ||||||||||||||||||||||||||||
| isoamylalcohol | 15.47 | 0.10 | 0.00 | 13.032 | 1.22 | 0.88 | 0.89 | ||||||||||||||||||||||||||||
| 1-pentanol | 16.98 | ND | - | 2.92 | 0.38 | 3.58 | 1.24 | ||||||||||||||||||||||||||||
| eptanol | 19.20 | 0.52 | 0.12 | 0.27 | 0.02 | 0.47 | 0.18 | ||||||||||||||||||||||||||||
| 3-hexen-1-ol | 20.81 | ND | - | 0.14 | 0.02 | 8.523 | 1.56 | ||||||||||||||||||||||||||||
| 2-nonanol | 20.87 | 2.60 | 0.67 | 0.46 | 0.10 | ND | - | ||||||||||||||||||||||||||||
| 1-octen-3-ol | 22.55 | ND | - | 1.39 | 0.46 | 0.32 | 0.19 | ||||||||||||||||||||||||||||
| 1-octanol | 24.93 | 0.08 | 0.05 | ND | - | ND | - | ||||||||||||||||||||||||||||
| 1-nonanol | 27.07 | ND | - | 1.18 | 0.09 | ND | - | ||||||||||||||||||||||||||||
| 3-decen-1-ol | 29.61 | ND | - | ND | - | 0.11 | 0.06 | ||||||||||||||||||||||||||||
| benzyl alcohol | 30.91 | ND | - | 0.32 | 0.01 | ND | - | ||||||||||||||||||||||||||||
| Total | 4.5 | 23.5 | 23.74 | ||||||||||||||||||||||||||||||||
| Furans | |||||||||||||||||||||||||||||||||||
| 2-methylfuran | 3.15 | 0.62 | 0.07 | ND | - | ND | - | ||||||||||||||||||||||||||||
| 4-ethylfuran | 4.21 | ND | - | ND | - | 5.22 | 1.43 | ||||||||||||||||||||||||||||
| Total | 0.62 | ND | 5.22 | ||||||||||||||||||||||||||||||||
| Terpenes | |||||||||||||||||||||||||||||||||||
| α-pinene | 6.03 | 47.951 | 2.30 | 6.478 | 0.04 | 6.235 | 0.73 | ||||||||||||||||||||||||||||
| thujene | 6.41 | 3.05 | 0.97 | 0.18 | 0.00 | 0.20 | 0.00 | ||||||||||||||||||||||||||||
| β-pinene | 9.63 | 1.42 | 0.64 | ND | - | 0.37 | 0.05 | ||||||||||||||||||||||||||||
| sabinene | 10.55 | 0.65 | 0.07 | ND | - | 0.20 | 0.18 | ||||||||||||||||||||||||||||
| β-myrcene | 13.33 | 2.07 | 0.09 | ND | - | 0.58 | 0.57 | ||||||||||||||||||||||||||||
| terpene | 14.39 | 2.83 | 0.25 | ND | - | 1.02 | 0.09 | ||||||||||||||||||||||||||||
| d-limonene | 14.69 | 0.99 | 0.13 | ND | - | 2.91 | 1.27 | ||||||||||||||||||||||||||||
| γ-terpinene | 16.40 | 0.46 | 0.00 | ND | - | 0.23 | 0.00 | ||||||||||||||||||||||||||||
| copaene | 23.38 | ND | - | 1.93 | 1.56 | ND | - | ||||||||||||||||||||||||||||
| isolongifolene | 23.89 | ND | - | 0.46 | 0.08 | 0.22 | 0.03 | ||||||||||||||||||||||||||||
| 4-thujanol | 24.58 | 0.51 | 0.08 | 0.27 | 0.00 | 0.12 | 0.03 | ||||||||||||||||||||||||||||
| linalool | 24.71 | ND | - | ND | - | 0.30 | 0.01 | ||||||||||||||||||||||||||||
| quinhydrone | 24.98 | 0.20 | 0.00 | 6.557 | 0.04 | 0.17 | 0.08 | ||||||||||||||||||||||||||||
| caryophillene | 25.61 | 0.07 | 0.02 | 0.18 | 0.07 | 0.08 | 0.27 | ||||||||||||||||||||||||||||
| 4-terpineol | 25.79 | 0.29 | 0.01 | 0.87 | 0.00 | 0.12 | 0.02 | ||||||||||||||||||||||||||||
| β-farnesene | 27.21 | ND | - | ND | - | ND | - | ||||||||||||||||||||||||||||
| verbenone | 27.64 | 0.24 | 0.03 | ND | - | 0.13 | 0.02 | ||||||||||||||||||||||||||||
| naphtalene | 28.90 | ND | - | ND | - | 0.22 | 0.04 | ||||||||||||||||||||||||||||
| sesquiphellandrene | 29.11 | ND | - | ND | - | 0.08 | 0.02 | ||||||||||||||||||||||||||||
| estragol | 30.05 | 0.14 | 0.00 | ND | - | ND | - | ||||||||||||||||||||||||||||
| Total | 67.57 | 17.19 | 13.33 | ||||||||||||||||||||||||||||||||
| Free fatty acids | |||||||||||||||||||||||||||||||||||
| acetic acid | 22.27 | 0.24 | 0.07 | 0.07 | 0.03 | ND | - | ||||||||||||||||||||||||||||
| formic acid | 23.46 | ND | - | 0.13 | 0.00 | ND | - | ||||||||||||||||||||||||||||
| propionic acid | 24.27 | ND | - | ND | - | ND | - | ||||||||||||||||||||||||||||
| pivalic acid | 25.21 | 0.04 | 0.00 | ND | - | 0.22 | 0.27 | ||||||||||||||||||||||||||||
| hexanoic acid | 30.34 | 0.36 | 0.11 | 0.09 | 0.02 | 0.11 | 0.01 | ||||||||||||||||||||||||||||
| heptanoic acid | 32.02 | ND | - | 0.14 | 0.05 | 1.34 | 0.18 | ||||||||||||||||||||||||||||
| octanoic acid | 33.06 | 0.06 | 0.02 | 0.16 | 0.02 | ND | - | ||||||||||||||||||||||||||||
| nonanoic acid | 34.13 | 0.19 | 0.05 | 0.31 | 0.01 | 0.14 | 0.06 | ||||||||||||||||||||||||||||
| Total | 0.88 | 0.91 | 1.81 | ||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||
| acetonitrile | 5.42 | 2.37 | 1.03 | 0.63 | 0.20 | 0.36 | 0.03 | ||||||||||||||||||||||||||||
| nicotinonitrile | 28.46 | 0.35 | 0.01 | 0.75 | 0.35 | 0.3 | 0.15 | ||||||||||||||||||||||||||||
| dimethyl sulfone | 31.24 | 0.37 | 0.02 | ND | - | ND | - | ||||||||||||||||||||||||||||
| Total | 3.08 | 1.37 | 0.65 | ||||||||||||||||||||||||||||||||
a Retention time; b Normalized amount of volatile compounds (percentage) (peak of volatile compound/total peak area of all volatile compounds) of O. sphegodes subsp. sphegodes, O. bertolonii subsp. benacensis and N. tridentata flowering orchid samples (n = 3); c Standard deviation (±); ND: not detected; The most representative compounds for all orchids species were labeled with a number.
Figure 2.
Volatile fingerprint of O. sphegodes subsp. sphegodes (A), O. bertolonii subsp. benacensis (B) and N. tridentata (C) picked flowering plants (the most representative compound peaks were labeled with numbers).
Comparing the VOC profiles of “in vivo” and picked flowering plant samples of the same orchid species we showed some differences in secondary metabolite composition. In particular we observed an increased content of phenols and alcohols in O. bertolonii subsp. benacensis and N. tridentata picked flowering orchid samples compared to living plants. Among these chemical classes we observed a large amount of 4-methylphenol in both orchid species (13,25% and 11,28% respectively) and of isoamylalcohol (13.03%) and 3-hexen-1-ol (8,52%) respectively in O. bertolonii subsp. benacensis and N. tridentata fingerprint.
An increase in hydrocarbons was also revealed in O. sphegodes subsp. sphegodes and O. bertolonii subsp. benacensis flowering plant samples and hexane, butane and nonane were considered the most representative compounds. Finally, we observed that flowering plant aromas showed a high content of terpenes. In particular in this case the most representative compounds were α-pinene (47.95%), thujene (3.05%) and cyclosativene (6.38%), followed in lesser amounts by β-myrcene (2.07%).
Collecting plant tissues and flowers definitely impacts on the volatile profiles, causing dramatically alterations if compared with in vivo and in situ VOC samples. These alterations can be related to the mechanical damage provoked by the collection of plants [27] and in particular in this study different proportions of the various terpenes were observed and a large increase of α-pinene was detected especially in O. sphegodes subsp. sphegodes fingerprint. These data were confirmed also by literature [27].
3. Experimental
3.1. Orchid Species Studied
Ophrys sphegodes Mill. subsp. sphegodes, commonly known as the Early Spider Orchid, is a very rare species that grows on alkaline and dry soils of Mediterranean coasts and mountain areas up to 1,200 m a.s.l. The plant is 25–50 cm tall and its inflorescence is formed by 4–6 flowers, which are characterized by yellow-green sepals and a velvety brown labellum with a distinctive H marking, so that the flowers very much resemble an arthropod and especially a spider.
Ophrys bertolonii subsp. benacensis (Reisigl) P. Delforge is a rare sub-endemic species that grows on basic and poor grasslands between 80 to 750 m a.s.l., in northern Italy. This orchid is 20–30 cm tall and, like O. sphegodes subsp. Sphegodes, its inflorescence is formed by 4–6 flowers. The flowers have white or lilac sepals, green veined, pinkish purple petals and the labellum is brown marked with bluish or reddish spots.
Neotinia tridentata (Scop.) R.M. Bateman, Pridgeon & M.W. Case is an Euro-Mediterranean species that grows in full sun on calcareous soils from sea level up to 1800 m a.s.l. The plant is 15–40 cm tall with a short, compact, ovoid inflorescence constituted by small, acuminate flowers. Sepals and petals are entirely lilac or pinkish purple veined, the labellum is trilobed, white to pale violet, marked with purple spots [28].
3.2. Orchid Population Sites Studied
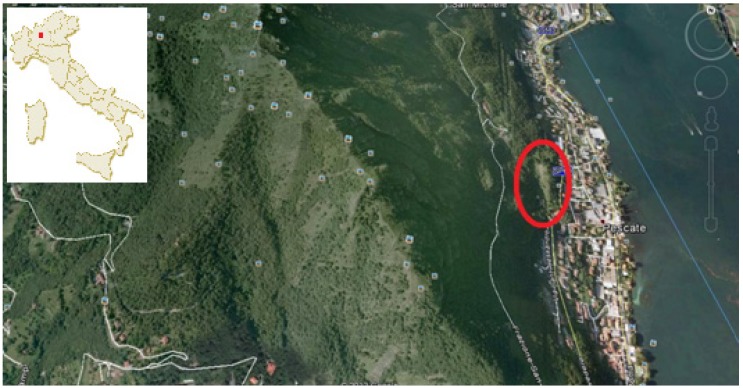
O. sphegodes subsp. sphegodes, O. benacensis and N. tridentata populations were identified [28] and sampled at flowering stage at the beginning of May 2013 in the area surrounding Prato Olivino, near Pescate, Lecco, Italy, (45°49’27.20” N; 9°23’53.54” E) located at an altitude of 280 m a.s.l. (Figure 3). According to the worldwide bioclimatic classification [29] the area belongs to the temperate oceanic bioclimate and to the low humid upper mesotemperate phytoclimatic belt. The habitat of orchids considered in this study is a semi-dry calcareous grassland (occasionally mown), belonging to the Festuco-Brometea Br.-Bl. & Tüxen ex Br.-Bl. 1949 class, Brometalia erecti Br.-Bl. 1936 order.
Figure 3.
Pescate, Lecco, North of Italy and detailed view of the site of orchid sampling, Prato Olivino (Map from Google earth).
Prato Olivino is a natural area near the Monte Barro natural park, which can been considered, in accordance with the Directive 92/42/CEE, as an important orchid site, due to the presence of rare and endemic species [30].
3.3. Headspace Solid Phase Microextraction (HS-SPME) of Volatile Compound Sampling from Living Orchid Plants (in Vivo)
At the beginning of May 2013, O. sphegodes subsp. sphegodes, O. benacensis and N. tridentata plants were sampled in triplicate, in vivo and in situ in order to evaluate the volatile organic compound (VOC) emitted by living plants. Each plant was enclosed in an customised aerated glass cage manufactured by COLAVER s.r.l. (Vimodrone, MI, Italy), into which a manual SPME holder was inserted to extract the headspace. Volatile compounds were collected using a 50/30 µm divinylbenzene/Carboxen™/polydimethylsiloxane (DVB/CAR/PDMS) StableFlex™ fiber (Supelco, Bellefonte, PA, USA). The fibre was exposed to the plant headspace for 4 h.
3.4. Headspace Solid Phase Microextraction (HS-SPME) of Volatile Compound Sampling from Flowering Orchid Plants
The HS-SPME extraction conditions were optimized in our previous study on the characterization of Achillea collina VOCs (selection of SPME fiber, sample amount, and extraction time, repeatability and precision of method) [31]. In this study flowering plant samples of all orchid species were picked and inserted into a 20 mL glass vial fitted with a cap equipped with a silicone/polytetrafluoroethylene septum (Supelco) in order to make the results comparable. Samples were prepared in triplicate. At the end of the sample equilibration period (1 h) a conditioned (1.5 h at 280 °C) 50/30 µm DVB/CAR/PDMS StableFlex™ fiber (Supelco) was exposed to the headspace of the sample for extraction (3 h) using a CombiPAL system injector autosampler (CTC Analytics, Zwingen, Switzerland). An extraction temperature of 30 °C was selected in order to prevent possible matrix alterations (oxidation of some compounds, particularly aldehydes) [32,33,34,35].
3.5. Gas Chromatography Mass Spectrometry Analysis of VOCs
HS-SPME analysis was performed using a Trace GC Ultra (Thermo-Fisher Scientific; Waltham, MA, USA) Gas Chromatograph coupled with a quadrupole Mass Spectrometer Trace DSQ (Thermo-Fisher Scientific; Waltham, MA, USA) and equipped with an Rtx-Wax column (30 m; 0.25 mm i.d.; 0.25 μm film thickness, Restek, PA, USA). The oven temperature program was: from 35 °C, hold 8 min, to 60 °C at 4 °C/min, then from 60 °C to 160 °C at 6 °C/min and finally from 160 °C to 200 °C at 20 °C /min. Carry over and peaks originating from the fiber were regularly assessed by running blank samples. After each analysis fibers were immediately thermally desorbed in the GC injector for 5 min at 250 °C to prevent contamination. The injections were performed in splitless mode (5 min). The carrier gas was helium at a constant flow of 1 mL·min−1. The transfer line to the mass spectrometer was maintained at 230 °C, and the ion source temperature was set at 250 °C. The mass spectra were obtained by using a mass selective detector with the electronic impact at 70 eV, a multiplier voltage of 1456 V, and by collecting the data at a rate of 1 scan·s−1 over the m/z range of 30–350. Compounds were identified by comparing the retention times of the chromatographic peaks with those of authentic compounds analyzed under the same conditions when available. The identification of MS fragmentation patterns was performed either by comparison with those of pure compounds or using the National Institute of Standards and Technology (NIST) MS spectral database. Volatile compound measurements from each headspace of orchid extracts were carried out by peak area normalization (expressed in percentage). All analyses were done in triplicate.
4. Conclusions
This study represented the first investigation regarding the VOC profile of different Italian populations of orchids using different pollinator attraction strategies, sampled in vivo and in situ. The results showed distinctive differences in volatile metabolite composition between orchids of the Ophrys and Neotinea genus. Moreover, a strong impact of the sampling methods on the volatile profiles, particularly regarding the different proportion of terpenes between picked flowering orchids and plants sampled in vivo and in situ, was observed. SPME could represent a good technique to analyze volatile compounds emitted by in vivo plants, sampled in situ, in a non-disruptive way, with potentially great advantages for phytochemical and ecophysiological studies, particularly regarding rare and/or protected plants, such as orchids.
Acknowledgments
This study was supported by “Accordo di Programma, affermazione in Edolo del Centro di Eccellenza Università della Montagna” MIUR-Università degli Studi di Milano, prot. No. 386 1293-05/08/2011. We are very grateful to Thomas Abeli, Università degli Studi di Pavia, for his information about orchid reproductive strategies.
Author Contributions
Alessandra Manzo, Sara Panseri, Ilda Vagge and Annamaria Giorgi, participated in designing the study. Alessandra Manzo and Sara Panseri conducted the study. Data was collected and analyzed by Ilda Vagge and Annamaria Giorgi. Manuscript was written by Alessandra Manzo, Sara Panseri, Ilda Vagge and Annamaria Giorgi.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples are not available from the authors.
References
- 1.Kaiser R. The Scent of Orchids: Olfactory and Chemical Investigation. Elsevier; Amsterdam, The Netherlands: 1993. [Google Scholar]
- 2.Knudsen J.T., Tollsten L. Trends in floral scent chemistry in pollination syndromes: Floral scent composition in moth pollinated taxa. Bot. J. Linn. Soc. 1993;113:263–284. doi: 10.1111/j.1095-8339.1993.tb00340.x. [DOI] [Google Scholar]
- 3.Anderson S., Dobson H.E. Behavioral foraging responses by the butterfly Heliconius melpomene to Lantana camara floral scent. J. Chem. Ecol. 2003;29:2303–2318. doi: 10.1023/A:1026226514968. [DOI] [PubMed] [Google Scholar]
- 4.Gumbert A. Color choices by bumble bees (Bombus terrestris): Innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 2000;48:36–43. doi: 10.1007/s002650000213. [DOI] [Google Scholar]
- 5.Dafni A. Mimicry and deception in pollination. Annu. Rev. Ecol. Syst. 1984;15:259–278. [Google Scholar]
- 6.Borg-Karlson A.K., Bergström G., Groth I. Chemical basis for the relationship between Ophrys orchids and their pollinators. I. Volatile compounds of Ophrys lutea and O. fusca as insect mimetic attractants/excitants. Chem. Scr. 1985;25:283–311. [Google Scholar]
- 7.Borg-Karlson A.K., Bergström G., Kullenberg B. Chemical basis for the relationship between Ophrys. orchids and their pollinators. II. Volatile compounds of O. insectifera and O. speculum as insect mimetic attractants/excitants. Chem. Scr. 1987;27:303–311. [Google Scholar]
- 8.Borg-Karlson A.K., Groth I., Agren L., Kullenberg B. Form-specific fragrances from Ophrys. insectifera L. (Orchidaceae) attract species of different pollinator genera: Evidence of sympatric speciation? Chemoecology. 1993;4:39–45. doi: 10.1007/BF01245895. [DOI] [Google Scholar]
- 9.Scopece G., Musacchio A., Widmer A., Cozzolino S. Patterns of reproductive isolation in Mediterranean deceptive orchids. Evolution. 2007;11:2623–2642. doi: 10.1111/j.1558-5646.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 10.Barták P., Bednář P., Cáp L., Ondráková L., Stranský Z. SPME-A valuable tool for investigation of flower scent. J. Sep. Sci. 2003;26:715–721. doi: 10.1002/jssc.200301381. [DOI] [Google Scholar]
- 11.Perraudin F., Popovici J., Bertrand C. Analysis of headspace-solid microextracts from flowers of Maxillaria tenuifolia Lindl. by GC-MS. Electron. J. Nat. Sub. 2006;1:1–5. [Google Scholar]
- 12.Arthur C.L., Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990;62:2145–2148. doi: 10.1021/ac00218a019. [DOI] [Google Scholar]
- 13.Zhang Z., Yang M.J., Pawliszyn J. Solid-Phase Microextraction. A Solvent-Free Alternative for Sample Preparation. Anal. Chem. 1994;66:844–853. doi: 10.1021/ac00089a001. [DOI] [Google Scholar]
- 14.Kullenberg B. Studies in Ophrys pollination. Zool. Bidr. Upps. 1961;34:1–340. [Google Scholar]
- 15.Kullenberg B. New observations on the pollination of Ophrys L. (Orchidaceae) Zoon. Suppl. 1973;1:9–14. [Google Scholar]
- 16.Paulus H.F., Gack C. Pollination of Ophrys (Orchidaceae) in Cyprus. Plant Syst. Evol. 1990;169:177–207. doi: 10.1007/BF00937674. [DOI] [Google Scholar]
- 17.Cozzolino S., D’Emerico S., Widmer A. Evidence for reproductive isolate selection in Mediterranean orchids: Karyotype differences compensate for the lack of pollinator specificity. Proc. Biol. Sci. 2004;271:S259–S262. doi: 10.1098/rsbl.2004.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce S., Ferrario A., Cerabolini B. Outbreeding and asymbiotic germination in the conservation of the endangered Italian endemic orchid Ophrys benacensis. Plant Biosyst. 2010;144:121–127. doi: 10.1080/11263500903192183. [DOI] [Google Scholar]
- 19.Melliou E., Kalpoutzakis E., Magiatis P., Tsitsa E. Composition of the Essential Oils of Orchis italica and Orchis quadripunctata from Greece. J. Essent. Oil Res. 2006;18:629–639. doi: 10.1080/10412905.2006.9699186. [DOI] [Google Scholar]
- 20.Tava A., Cecotti R., Confalonieri M. Characterization of the volatile fraction of Nigritella nigra (L.) Rchb. F. (Orchidaceae.), a rare species from the Central Alps. J. Essent. Oil Res. 2010;24:39–44. [Google Scholar]
- 21.Ono T., Miyazawa M. Headspace constituents of flowers of Neofinetia falcata. Nat. Prod. Lett. 1999;13:53–57. doi: 10.1080/10575639908048491. [DOI] [Google Scholar]
- 22.Junsrigival J., Sonngsak T., Kirdmanee C., Chansakaow S. Determination of volatile constituents of Thai fragrance orchids by gas chromatography-mass spectrometry with solid-phase microextraction. Chiang Mai Univ. J. Nat. Sci. 2013;12:43–57. [Google Scholar]
- 23.Da Silva U.F., Borba U.L., Semir J., Marsaioli A.J. A simple solid injection device for the analysis of Bulbophyllum. (Orchidaceae) volatiles. Phytochemistry. 1999;50:31–34. doi: 10.1016/S0031-9422(98)00459-2. [DOI] [Google Scholar]
- 24.Francke W. Convergency and diversity in multicomponent insect pheromones. Adv. Invertebr. Reprod. 1986;1:327–336. [Google Scholar]
- 25.Delforge P. Orchids of Europe, North Africa and the Middle East. 3rd ed. A&C Black Publishers Ltd.; London, UK: 2006. [Google Scholar]
- 26.Salzmann C., Brown A., Schiestl F. Floral scent in food-deceptive orchids: Species specificity and sources of variability. Plant Biol. 2007;9:720–729. doi: 10.1055/s-2007-965614. [DOI] [PubMed] [Google Scholar]
- 27.Schiestl F.P., Ayasse M., Paulus H.F., Erdmann D., Francke W. Variation of floral scent emission and post pollination changes in individual flowers of Ophrys sphegodes subsp. sphegodes (Orchidaceae.) J. Chem. Ecol. 1997;23:2881–2895. doi: 10.1023/A:1022527430163. [DOI] [Google Scholar]
- 28.Pignatti S. Flora d’Italia. Edagricole; Bologna, Italy: 1982. [Google Scholar]
- 29.Rivas-Martinez S. Global Bioclimatics (Clasificación Bioclimática de la Tierra) [(accessed on 4 April 2014)]. Available online: http://www.globalbioclimatics.org/book/bioc/global_bioclimatics-2008_00.htm.
- 30.Pierce S., Ceriani R.M., Villa M., Cerabolini B. Quantifying relative extinction risks and targeting intervention for the orchid flora of a natural park in the European prealps. Conserv. Biol. 2006;20:1804–1810. doi: 10.1111/j.1523-1739.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 31.Giorgi A., Madeo M., Speranza G., Cocucci M. Influence of environmental factors on composition of phenolic antioxidants of Achillea collina Becker ex Rchb. Nat. Prod. Res. 2010;24:1546–1559. doi: 10.1080/14786419.2010.490656. [DOI] [PubMed] [Google Scholar]
- 32.Panseri S., Catalano A., Giorgi A., Arioli F., Procopio A., Britti D., Chiesa L.M. Occurrence of pesticide residues in Italian honey from different areas in relation to its potential contamination sources. Food Control. 2013;38:150–156. [Google Scholar]
- 33.Panseri S., Manzo A., Chiesa L.M., Giorgi A. Melissopalynological and volatile compounds analysis of buckwheat honey from different geographical origins and their role in botanical determination. J. Chem. 2013;2013:904202:1–904202:11. [Google Scholar]
- 34.Giorgi A., Panseri S., Mattara M.S., Andreis C., Chiesa L.M. Secondary metabolites andantioxidant capacities of Waldheimia glabra (decne.) regel from Nepal. J. Sci. Food Agric. 2013;93:1026–1034. doi: 10.1002/jsfa.5839. [DOI] [PubMed] [Google Scholar]
- 35.Giorgi A., De Marinis P., Granelli G., Chiesa L.M., Panseri S. Secondary metabolite profile, antioxidant capacity, and mosquito repellent activity of Bixa orellana from Brazilian Amazon region. J. Chem. 2013;2013:409826:1–409826:10. [Google Scholar]