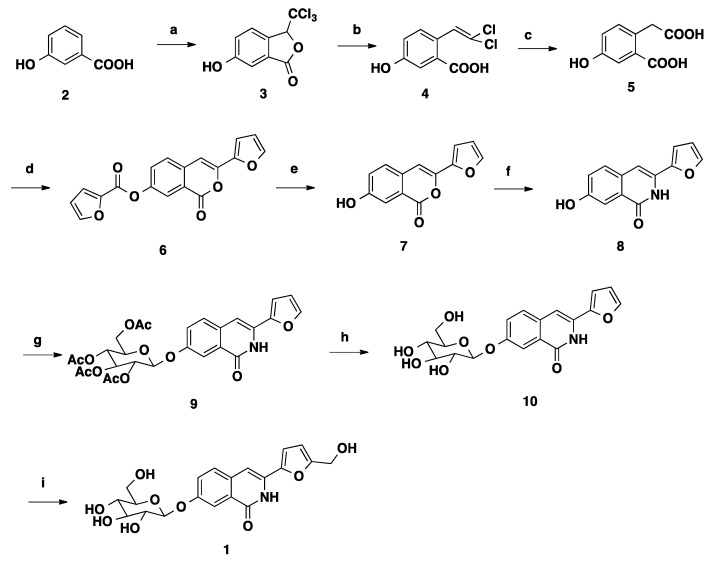
Scheme 1.
Synthesis route of 3-(5-(hydroxymethyl)furan-2-yl)-7-(((2S,3R,5S,6R)-3,4,5- trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)isoquinolin-1(2H)-one (1).
Reagents and conditions: (a) Chloral hydrate (1 eq), conc. H2SO4, room temperature, 16 h; (b) Zn, HOAc, room temperature, 30 min; (c) conc. H2SO4, room temperature, 30 min; (d) 2-furoyl chloride, 200 °C, 4 h; (e) LiOH (5 eq), THF, water, room temperature, 30 min; (f) NH4OH, EtOH, 120 °C, 6 h; (g) tetraacetyl-d-glucose 2,2,2-trichloroacetimidate (2 eq), CH2Cl2, BF3-Et2O, 0 °C, 2 h; (h) CH3ONa/CH3OH, room temperature, 20 min; (i) aqueous formaldehyde, AlCl3, 90 °C, 3 h.