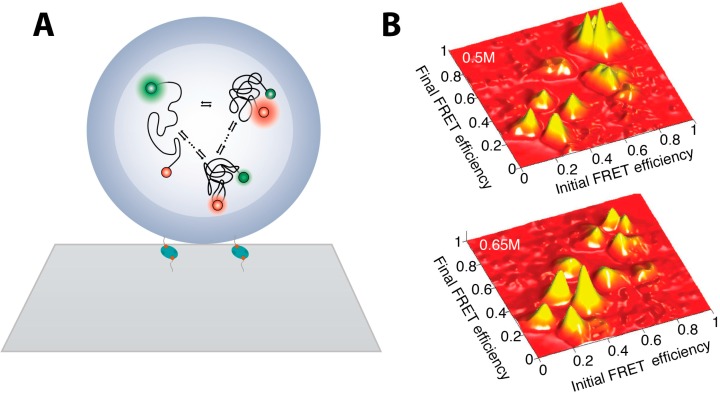
Figure 2.
smFRET studies of adenylate kinase enzymes folding (A) Individual AK enzymes, double labeled for smFRET measurements, were encapsulated inside liposomes minimizing interaction with hard matter allowing unbiased measurements of the folding landscape. Liposomes are tethered on glass microscope surface via biotin-avidin interaction; (B) Transition density plots showing the preferred transition pathway of folding to depend on denaturant concentrations. As the denaturant concentration increases more transition between lower FRET states occur and the fraction of sequential—between states of similar FRET intensity—increases (Figure 2 is adapted from [74] with permission).