Figure 1.
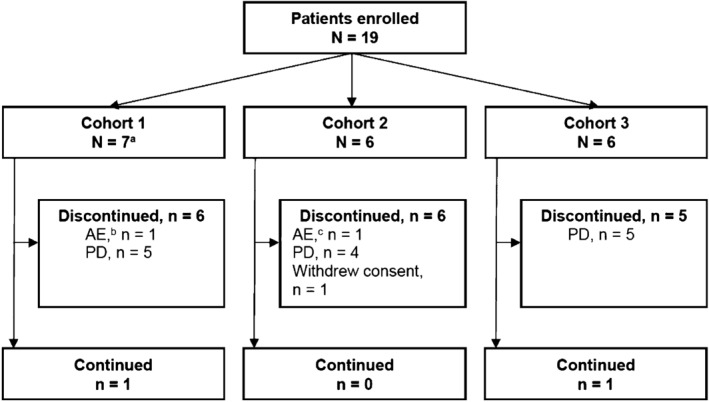
Patient disposition. aOne patient in Cohort 1 was replaced as per protocol because of an IRR; bDue to a treatment‐related adverse event of IRR during Cycle 1, Day 1; cDue to a treatment‐related adverse event of Grade 3 left ventricular dysfunction on Day 35 after completion of Cycle 6. AE, adverse event; IRR, infusion‐related reaction; PD, progressive disease
