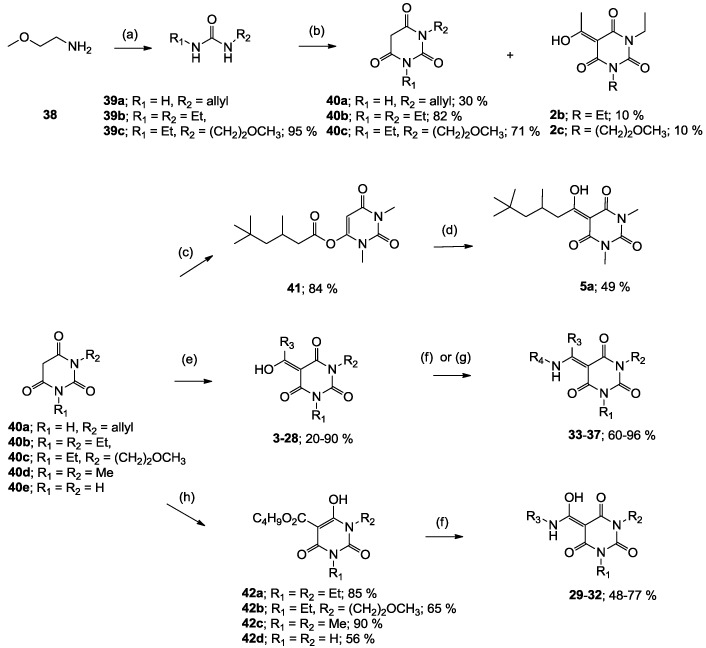
Scheme 1.
Synthesis of barbituric acid analogues. Reaction conditions; (a) ethyl isocyanate (1.0 eq), CH2Cl2, 0 °C; (b) malonic acid (1.0 eq), acetic acid, acetic anhydride, 60–90 °C; (c) 3,5,5-trimethylhexanoyl chloride (1.1 eq), triethylamine (1.2 eq), CH2Cl2, r.t.; (d) DMAP (1.2 eq), CH2Cl2, r.t.; (e) R3CO2H (1.1 eq), DCC (1.1 eq), DMAP (1.2 eq), CH2Cl2, r.t.; (f) RNH2 (1.0 eq), toluene, reflux; (g) RNH2 (1.1 eq), CH3OH, reflux; (h) butyl chloroformate (1.2 eq), DMAP (2.2 eq), CH2Cl2, r.t.; Abbreviation; DCC; N,N′-dicyclohexylcarbodiimide, DMAP; 4-(dimethylamino)pyridine.