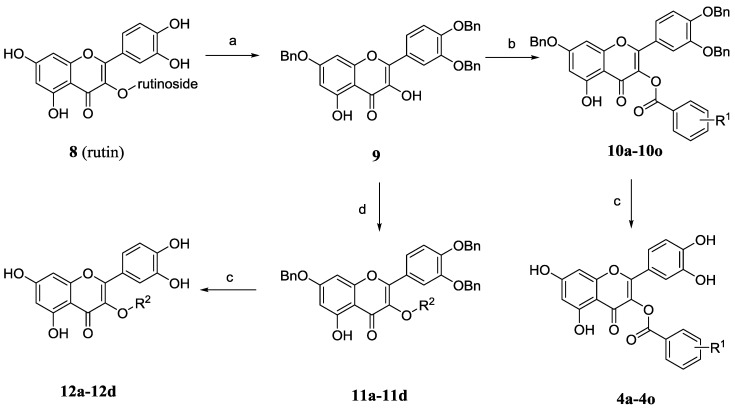
Scheme 2.
Synthesis of the quercetin-3-O-substituted benzoic acid esters and 3-O-substituted quercetin derivatives.
| R1 | R1 | R1 | R2 | ||||
|---|---|---|---|---|---|---|---|
| 4a | H | 4f | 3''-F | 4k | 4''-F | 12a | Me |
| 4b | 2''-F | 4g | 3''-NH2 | 4l | 4''-NH2 | 12b | i-Pr |
| 4c | 2''-NH2 | 4h | 3''-OCH3 | 4m | 4''-OCH3 | 12c | (CH2)2OH |
| 4d | 2''-OCH3 | 4i | 3''-CN | 4n | 4''-CN | 12d | (CH2)3CN |
| 4e | 2''-CN | 4j | 3''-Cl | 4o | 4''-Cl |
Reagents and conditions: (a) K2CO3, BnBr, DMF, 60 °C, 3 h, then HCl con., ethanol, 70 °C, 2 h, 85%; (b) R1-PhCOOH, EDCI, DMAP, DMF, rt, overnight; (c) H2, Pd/C, 1,4-dioxane/ethanol, rt, 6 h, 40%–79% (two steps); (d) R2Br or R2I, K2CO3, DMF, 40 °C, 3 h.