Table 2.
Structural and chromatographic characteristics of investigated phenolic compounds.
| Structural Formula | Compounds | R1 | R2 | λmax (nm) | Molecular Weight | ESI-MS2 (m/s) |
|---|---|---|---|---|---|---|
HCAs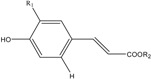
|
3-p-CoQA | H | 3-quinic acid | 226.1, 310.3 | 338 | 336.9, 163.1, 119.0 |
| 5-CQA | OH | 5-quinic acid | 241.4, 324.6 | 354 | 353.0, 191.0, 135.1 | |
| 4-CQA | OH | 4-quinic acid | 240.2, 327.0 | 354 | 353.0, 190.8, 179.1 | |
| 3-CQA | OH | 3-quinic acid | 241.4, 324.6 | 354 | 353.0, 191.0, 135.1 | |
| 5-FQA | OCH3 | 5-quinic acid | 216.6, 325.8 | 368 | 367.0, 191.0, 85.0 | |
Flavonols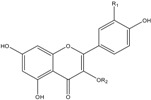
|
Q-3-Gal | OH | galactoside | 255.6, 352.8 | 464 | 463.3, 301.1, 300.1 |
| Q-3-Glu | OH | glucoside | 255.6, 352.8 | 464 | 463.3, 301.2, 300.1 | |
| Q-3-Rha | OH | rhamnoside | 255.6, 348.0 | 448 | 447.2, 301.2, 300.3 | |
| K-3-Gal | H | galactoside | 265.1, 346.8 | 448 | 447.2, 285.0, 284.2 | |
| K-3-Rha | H | rhamnoside | 263.9, 341.1 | 432 | 431.3, 285.2, 284.2 | |
| K-3-Glu | H | glucoside | 253.9, 349.2 | 448 | 447.2, 285.2, 284.1 |
