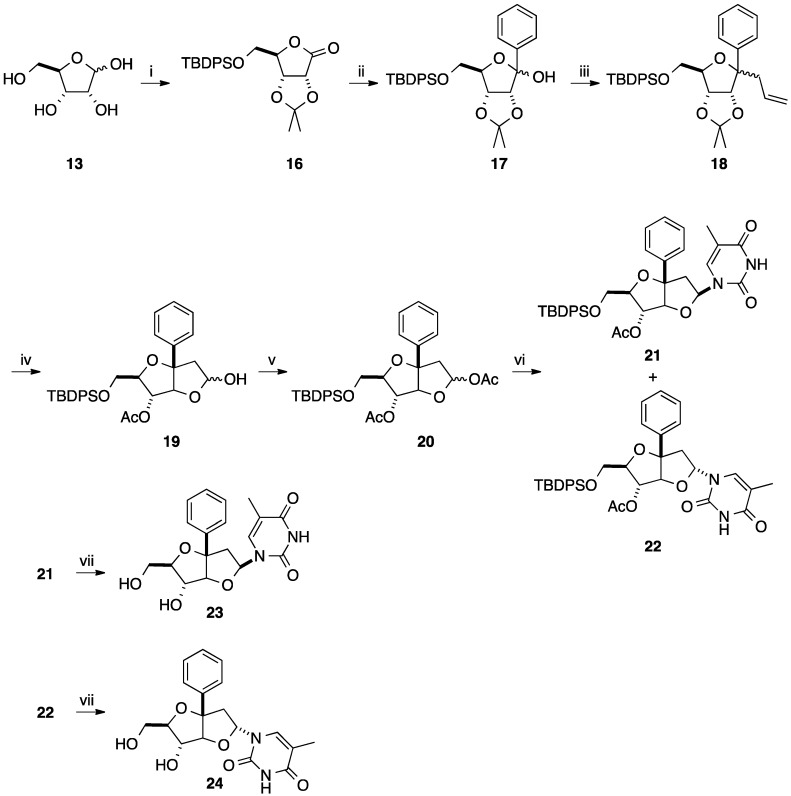
Scheme 4.
Synthesis of thymidine analogues 23 and 24.
Reagents and Conditions: (i) (a) Acetone, H+; (b) TBDPSCl, TEA, DMAP, CH2Cl2; (c) PCC, CH2Cl2, 77% for the three steps; (ii) PhLi, THF, −78 °C, 2 h, 79%; (iii) CH2=CHCH2TMS, ZnBr2, CH3NO2, 0 °C then rt, 2 h, 82%; (iv) (a) aq. OsO4, NaIO4, pyridine, rt, 30 h; (b) H2SO4 (5%), THF, 60 °C, 6 h, 28% for the two steps; (v) Ac2O, pyridine, 0 °C, 39 h, 90%; (vi) HDMS, TMSCl, SnCl4, thymine, CH3CN, 50 °C, 4 h, 21: 42%; 22: 37%; (vii) (a) TBAF, THF, rt, 2 h; (b) NaOH, THF, MeOH, 0 °C, 1 h, 23: 71%; 24: 47% for the two steps.