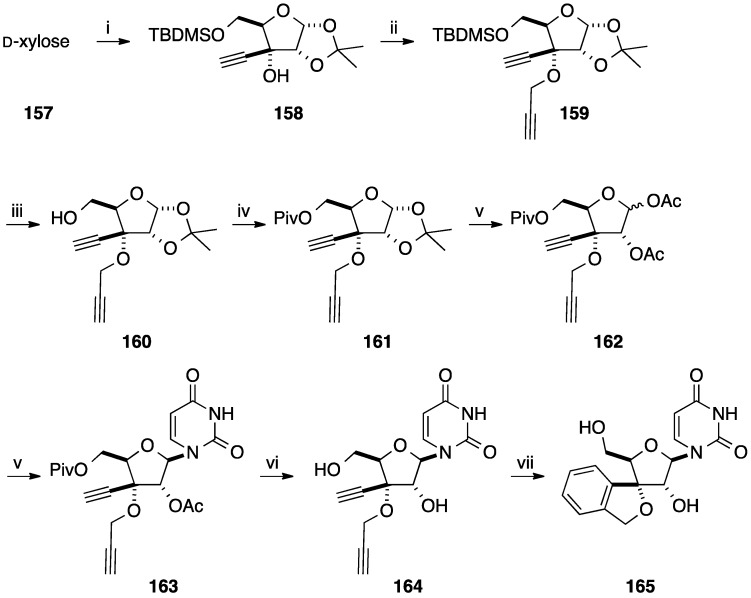
Scheme 20.
Synthesis of the 3'-C-spiro uridine analogue 165.
Reagents and Conditions: (i) Reference [42]; (ii) NaH, propargyl bromide, THF, 0 °C to rt, 3 h, 83%; (iii) TBAF, THF, rt, 8 h, 98%; (iv) PivCl, TEA, DMAP, CH2Cl2, 0 °C to rt, 6 h, 81%; (v) (a) Aq AcOH (60%), reflux, 2 h; (b) Ac2O, TEA, DMAP, CH2Cl2, 87% for the two steps; (vi) uracil, BSA, TMSOTf, CH3CN, 50 °C, 2 h, 75%; (vii) MeONa, MeOH, rt, 20 min, 78%; (viii) Cp*RuCl(cod) (5 mol %), C2H4Cl2, EtOH, rt, 4–6 h, 79%.