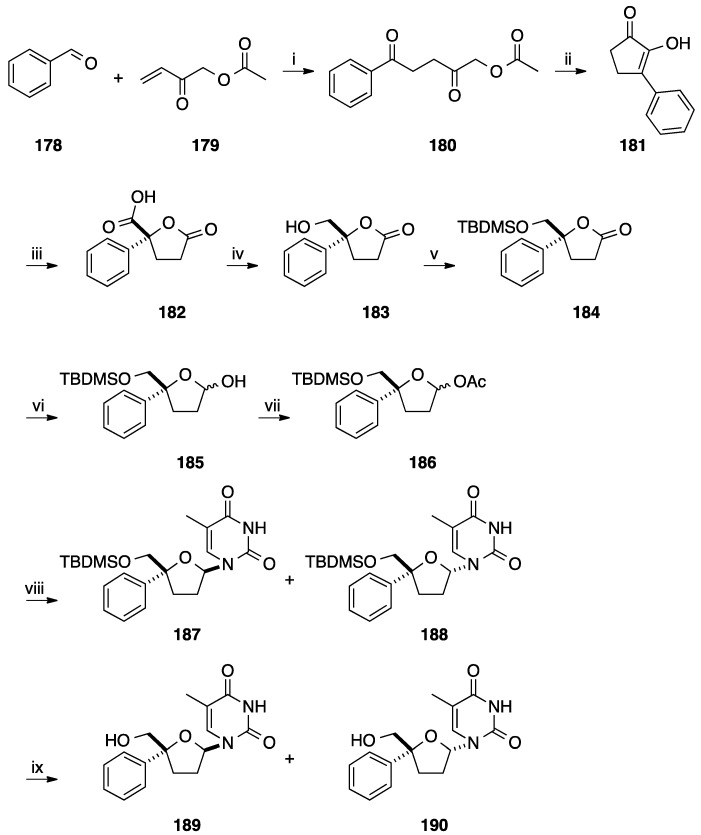
Scheme 21.
Synthesis of the 4'-C-phenyl thimidine analogues 189 and 190.
Reagents and Conditions: (i) 3-Benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride, TEA, dioxane, 70–80 °C, 15 h, 71%; (ii) MeONa, MeOH, reflux, 40%; (iii) Ti(Oi-Pr)4, (+)-diethyl tartrate, t-BuOOH, CH2Cl2, −20 °C, 114 h, 36% (ee 86%); (iv) BH3.SMe2, THF, 86%; (v) TBDMSCl, imidazole, CH2Cl2, 100%; (vi) DIBAL-H, toluene, 92%; (vii) Ac2O, TEA, CH2Cl2, 90%; (viii) thymine, BSA, TMSOTf, CH3CN, 88% (187:44%, 188:44%); (ix) TBAF, THF, 100%.