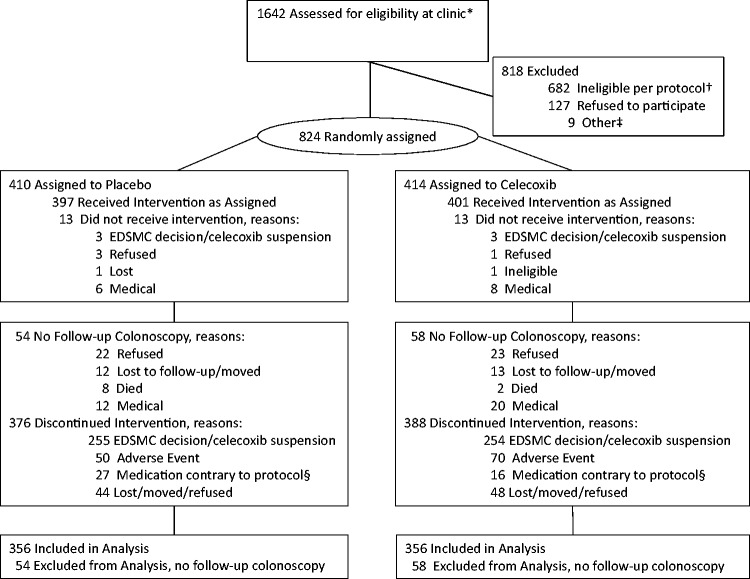
Figure 1.
Consort diagram. *Participants were recruited through clinical centers in Arizona, Colorado, Texas, and New York following ambulatory colonoscopies conducted at high-volume endoscopy facilities. †Not eligible per protocol, including medical conditions (264), medication use (145), regular high-dose aspirin/NSAID use (171), clinical lab results (8), supplemental selenium use (23), other (71). ‡Other, including five lost to follow-up or moved, one deceased, three toxicity during placebo run-in. §Medications contrary to protocol include: Celebrex, VIOXX, other NSAIDs, supplemental selenium, coumadin.