Table 1.
Data for amides derived from cinnamic acid and benzoic acid.
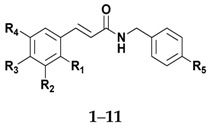 |
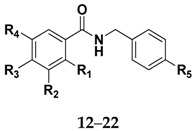 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | R5 | Molecular Formula | Reaction Time (h) | Yield (%) |
| 1 | - | - | - | - | Cl | C16H14ClNO | 3 | 75 |
| 2 | - | OH | OH | - | Cl | C16H14ClNO3 | 7 | 70 |
| 3 | - | OMe | OH | - | Cl | C17H16ClNO3 | 4 | 81 |
| 4 | - | - | OMe | - | Cl | C17H16ClNO2 | 2 | 91 |
| 5 | OH | - | - | - | Cl | C16H14ClNO2 | 5 | 79 |
| 6 | - | OH | - | - | Cl | C16H14ClNO2 | 4 | 76 |
| 7 | - | - | OH | - | Cl | C16H14ClNO2 | 3 | 63 |
| 8 | - | - | Cl | - | Cl | C16H13Cl2NO | 2 | 71 |
| 9 | - | OMe | OH | OMe | Cl | C18H18ClNO4 | 6 | 60 |
| 10 | NO2 | - | - | - | Cl | C16H13ClN2O3 | 2 | 79 |
| 11 | - | OMe | OMe | OMe | Cl | C19H20ClNO4 | 3 | 86 |
| 12 | - | - | - | - | Cl | C14H12ClNO | 3 | 65 |
| 13 | - | - | C6H5 | Cl | C20H16ClNO | 6 | 56 | |
| 14 | - | OH | OH | OH | Cl | C14H12ClNO4 | 3 | 21 |
| 15 | - | OMe | OH | - | Cl | C15H14ClNO3 | 6 | 44 |
| 16 | - | OMe | OH | OMe | Cl | C16H16ClNO4 | 3 | 50 |
| 17 | - | - | OH | - | Cl | C14H12ClNO2 | 3 | 73 |
| 18 | - | C(CH3)3 | OH | C(CH3)3 | Cl | C22H28ClNO2 | 3 | 54 |
| 19 | - | OH | - | OMe | Cl | C15H14ClNO3 | 6 | 60 |
| 20 | - | Me | - | NO2 | Cl | C15H13ClN2O3 | 4 | 41 |
| 21 | - | OMe | OH | - | F | C15H14FNO3 | 2 | 27 |
| 22 | - | OMe | OH | - | Br | C15H14BrNO3 | 2 | 63 |
