Abstract
A series of novel benzohydrazide derivatives containing dihydropyrazoles have been synthesized as potential epidermal growth factor receptor (EGFR) kinase inhibitors and their biological activities as potential antiproliferative agents have been evaluated. Among these compounds, compound H20 exhibited the most potent antiproliferative activity against four cancer cell line variants (A549, MCF-7, HeLa, HepG2) with IC50 values of 0.46, 0.29, 0.15 and 0.21 μM respectively, which showed the most potent EGFR inhibition activities (IC50 = 0.08 μM for EGFR). Molecular modeling simulation studies were performed in order to predict the biological activity and activity relationship (SAR) of these benzohydrazide derivatives. These results suggested that compound H20 may be a promising anticancer agent.
Keywords: benzohydrazide derivatives, EGFR inhibitor, antiproliferative activity, cytotoxicity, molecular docking
1. Introduction
Cancer has become one of the most serious diseases which represents a great threat to human health around the world [1]. Although there are currently a large number of anticancer drugs, more and more people continue to die of cancer [2]. Epidermal growth factor receptor (EGFR) plays important roles in human cancer. As a member of the HER family, EGFR is a tyrosine kinase receptor which plays an essential role in normal cell growth and differentiation, and is involved in tumor proliferation and survival [3,4]. The HER family comprises four members: EGFR (HER1/ErbB-1), ErbB-2 (HER2/neu), ErbB-3 (HER3), and ErbB-4 (HER4) [5]. EGFR is one of the most important targets in current cancer research and its over-expression or abnormal activation often causes cell malignant transformation [6]. The over-expression of EGFR has been observed in many solid tumors, such as colon [7], ovarian [8], breast and non-small cell lung cancer (NSCLC) [9]. Therefore, EGFR inhibition has been developed as one of the most efficient strategies for cancer therapy.
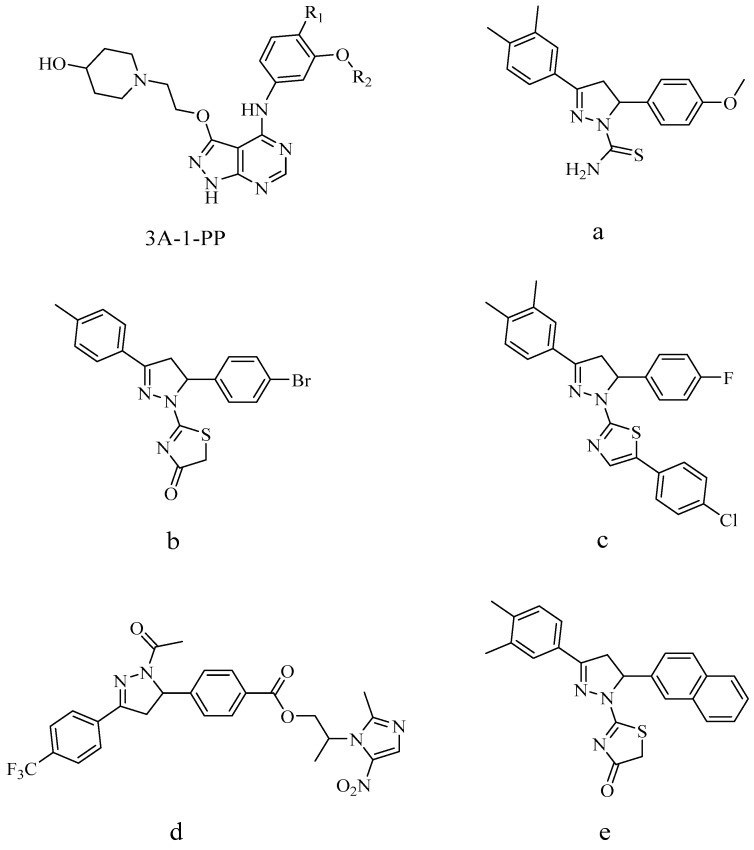
Dihydropyrazole, a small bioactive molecule, is a prominent structural motif found in numerous pharmaceutically active compounds. Some representatives had been demonstrated to have important biological activities such as antiviral/antitumor [10], antibacterial [11], fungistatic [12], antihyperglycemic activity [13], antimalarial [14] and antitubercular effects [15]. Biological evaluation indicated that some of the synthesized compounds were potent inhibitors of EGFR. Some novel compounds containing the dihydropyrazole skeletons were reported as potent anticancer agents against EGFR tyrosine kinase. It had been reported that anticancer compounds such as 3-alkoxy-1H-pyrazolo[3,4-d]pyrimidines analogues (3A-1-PP, Figure 1) showed potent EGFR kinase inhibitory activity, with IC50 values reaching single digit nanomolar values [16]. Compounds a, b, c and d (Figure 1) displayed the most potent EGFR inhibitory activity, with IC50 values of 0.07, 0.24, 0.06 and 0.26 μM respectively, which were comparable to the positive control erlotinib (IC50 = 0.03 μM) [17,18,19,20]. Compound e containing the dihydropyrazole and naphthalene ring showed the highest inhibitory EGFR activity (IC50 = 0.12 μM) [21].
Figure 1.
Chemical structures of some reported compounds.
Some naphthalene derivatives have been reported as potent microtubule inhibitors, apoptosis inducers, glutamamide analogues or P-glycoprotein inhibitors [22,23]. On the other hand, the naphthalene ring can be recognized in various biologically active compounds with clinical applications. Naftifine and terbinafine, allylamine antifungal agents, are widely used for the treatment of fungal infections [24,25,26]. Propranolol is an anti-angina drug, duloxetine is used for the treatment of depression, nafimidone and its oxime ester derivative can be further developed for the treatment of epilepsy [27]. Therefore, preparation and extensive biological evaluations on naphthalene derivatives have continuously attracted our attention [28]. Besides, benzohydrazides are reported to possess a wide variety of biological activities like antiglycation [29], antioxidant [30], antileishmanial [22], antibacterial [31], antifungal [23], antitumor [32] and anticonvulsant [24]. Benzothiazole Schiff bases with diverse biological activities have also been reported [26].
The three combined substructures—the dihydropyrazole along with the naphthalene ring and benzohydrazides—might exhibit synergistic anticancer effects. All of these facts encouraged us to integrate these three moieties and screen new benzohydrazide derivatives containing dihydropyrazoles as potential EGFR inhibitory agents. Herein we disclose the design and synthesis of some novel benzohydrazide derivatives containing dihydropyrazoles as potential EGFR kinase inhibitors, and their antitumor activity against the A549 (human lung cancer), MCF-7 (human breast cancer), HeLa (human cervical cancer) and HepG2 (human hepatocellular cancer) cancer cell lines.
2. Results and Discussion
2.1. Chemistry
A series of novel benzohydrazide derivatives containing dihydropyrazole moieties were synthesized by the routes outlined in Scheme 1. The structures of the compounds are listed in Table 1. Methyl 4-hydrazinylbenzoate (B) was synthesized from 4-hydrazinylbenzoic acid in methanol at room temperature. The diverse substituted chalcones E1–E16 were obtained by stirring substituted acetophenones and naphthaldehyde in ethanol at room temperature for 6–8 h. The cyclization different chalcones and methyl 4-hydrazinylbenzoate in refluxing ethanol gives compounds F1–F16.
Scheme 1.
The synthetic routes to compounds H1–H32. Reagents and conditions: (i) MeOH, SOCl2, room temperature, 20–24 h; (ii) EtOH, KOH, room temperature, 6–8 h; (iii) EtOH, HAc, 80 °C, 20–24 h; (iv) MeOH, N2H4·H2O (80%), DMF, 70 °C, 20–24 h; (v) RCHO, EtOH, HOAc, room temperature, 6–8 h.
Table 1.
Structures of compounds H1–H32.
| Compound | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| H1 | H | H | H | H | OCH3 | H |
| H2 | CH3 | H | H | H | OCH3 | H |
| H3 | H | CH3 | H | H | OCH3 | H |
| H4 | H | H | CH3 | H | OCH3 | H |
| H5 | H | H | CH2CH3 | H | OCH3 | H |
| H6 | H | H | OCH3 | H | OCH3 | H |
| H7 | H | OCH3 | OCH3 | OCH3 | OCH3 | F |
| H8 | H | H | OCH2CH3 | H | OCH3 | H |
| H9 | F | H | H | H | OCH3 | H |
| H10 | H | F | H | H | OCH3 | H |
| H11 | H | H | F | H | OCH3 | H |
| H12 | H | F | H | F | OCH3 | H |
| H13 | H | Cl | H | H | OCH3 | H |
| H14 | H | H | Cl | H | OCH3 | H |
| H15 | H | Cl | Cl | H | OCH3 | H |
| H16 | H | H | Br | H | OCH3 | H |
| H17 | H | H | H | H | H | OCH3 |
| H18 | CH3 | H | H | H | H | OCH3 |
| H19 | H | CH3 | H | H | H | OCH3 |
| H20 | H | H | CH3 | H | H | OCH3 |
| H21 | H | H | CH2CH3 | H | H | OCH3 |
| H22 | H | H | OCH3 | H | H | OCH3 |
| H23 | H | OCH3 | OCH3 | OCH3 | F | OCH3 |
| H24 | H | H | OCH2CH3 | H | H | OCH3 |
| H25 | F | H | H | H | H | OCH3 |
| H26 | H | F | H | H | H | OCH3 |
| H27 | H | H | F | H | H | OCH3 |
| H28 | H | F | H | F | H | OCH3 |
| H29 | H | Cl | H | H | H | OCH3 |
| H30 | H | H | Cl | H | H | OCH3 |
| H31 | H | Cl | Cl | H | H | OCH3 |
| H32 | H | H | Br | H | H | OCH3 |
Compounds F1–F16 further reacted with excess hydrazine hydrate (80%) to give the requisite intermediate G1–G16. The desired compounds H1–H32 were generated by the reaction of compounds G1–G16 with substituted benzaldehydes in ethanol for 6–8 h. Purified compounds H1–H32 were finally obtained by chromatography. All synthesized compounds H1–H32 gave satisfactory analytical and spectroscopic data in full accordance with their depicted structures, and other data published in a Chinese patent [33].
2.2. Bioassays
2.2.1. Antiproliferative Assay
The antiproliferative activities of the newly synthesized derivatives H1–H32 were evaluated under identical conditions by the MTT assay against four cultured cell lines (A549, MCF-7, HeLa and HepG2) with erlotinib as control. The IC50 values of the compounds against these human cancer cells are summarized in Table 2. As shown, the results revealed that all of the target compounds exhibited significant antiproliferative activities, ranging from 0.15 to 100 μM.
Table 2.
In vitro antiproliferative activities (IC50, μM a) of all compounds against tumor cell lines.
| Compounds | A549 b | MCF-7 b | HeLa b | HepG2 b |
|---|---|---|---|---|
| H1 | 14.52 | 20.96 | 22.47 | 28.76 |
| H2 | 1.41 | 1.52 | 0.60 | 0.67 |
| H3 | 3.02 | 2.89 | 2.45 | 3.54 |
| H4 | 0.43 | 0.69 | 0.45 | 0.51 |
| H5 | 5.49 | 7.72 | 8.71 | 6.16 |
| H6 | 8.32 | 10.17 | 13.16 | 11.45 |
| H7 | 78.32 | 95.32 | >100 | >100 |
| H8 | 10.71 | 15.87 | 17.08 | 19.75 |
| H9 | 50.65 | 70.36 | 63.78 | 76.48 |
| H10 | 58.59 | 80.12 | 84.12 | 92.50 |
| H11 | 45.16 | 60.79 | 56.21 | 60.86 |
| H12 | 41.48 | 55.64 | 50.34 | 52.30 |
| H13 | 35.25 | 51.28 | 46.38 | 45.37 |
| H14 | 30.14 | 42.46 | 37.43 | 40.20 |
| H15 | 26.57 | 35.24 | 33.62 | 38.93 |
| H16 | 21.78 | 31.85 | 26.51 | 34.19 |
| H17 | 13.30 | 18.39 | 20.73 | 26.67 |
| H18 | 1.22 | 1.63 | 0.78 | 0.56 |
| H19 | 2.24 | 2.45 | 1.19 | 3.26 |
| H20 | 0.46 | 0.29 | 0.15 | 0.21 |
| H21 | 4.54 | 6.33 | 7.93 | 5.06 |
| H22 | 7.12 | 9.69 | 12.31 | 10.54 |
| H23 | 75.66 | 93.23 | 94.10 | 89.47 |
| H24 | 9.92 | 13.79 | 15.28 | 18.56 |
| H25 | 48.55 | 65.53 | 61.87 | 71.10 |
| H26 | 56.39 | 77.49 | 85.15 | 80.07 |
| H27 | 44.11 | 58.98 | 54.22 | 62.94 |
| H28 | 39.42 | 56.14 | 47.67 | 48.06 |
| H29 | 33.25 | 50.88 | 42.95 | 40.99 |
| H30 | 28.64 | 40.40 | 35.34 | 38.20 |
| H31 | 24.77 | 34.44 | 31.82 | 36.33 |
| H32 | 19.71 | 30.25 | 25.55 | 32.91 |
| Erlotinib | 0.20 | 0.12 | 0.17 | 0.10 |
a Antiproliferation activity was measured using the MTT assay. Values are the average of six independent experiments run in triplicate. Variation was generally 5%–10%; b Cancer cells kindly supplied by State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University.
Among them, compound H20, which had IC50 values of 0.46, 0.29, 0.15 and 0.21 μM against A549, MCF-7, HeLa and HepG2, respectively, displayed the most potent antiproliferative activity, comparable to that of the positive control drug erlotinib (with corresponding IC50 values of 0.20, 0.12, 0.17 and 0.10 μM). These results suggested that compound H20 is more potent than the other compounds overall. Modification of substituents such as methyl, halogen, methoxyl, ethyl and ethoxy was performed to explore the structure-activity relationships of these compounds. Compounds bearing one methyl group at the para-position in the A ring showed stronger antiproliferative activity (the IC50 values of H4 and H20: 0.45 and 0.21 μM against HeLa cells) than those with hydrogen (H1 and H17), fluorine (H11 and H27), chlorine (H14 and H30), bromine (H16 and H32), ethyl (H5 and H21), ethoxy (H8 and H24) and methoxyl (H6 and H22) substituents, in the order of Me > H > Br > Cl > F, Me > OMe and Et > OEt. This means that compounds with electron-donating groups at the para-position of the A ring had better antiproliferative activity than those with electron-withdrawing groups. A comparison of the composition on the A ring was as follows: for compounds H2–H4, H9–H11, H13–H14, H18–H20, H25–H27 and H29–H30, the potency order of substituent groups on the A ring was found to be para > ortho > meta. Compounds H9–H15 and H25–H31 with halogen groups on the A ring exhibited antiproliferative activities in the order of disubstituted > monosubstituted. Interestingly, compounds H6 and H22 with one methoxyl group on the A ring showed stronger antiproliferative activities than compounds H7 and H23 with three methoxyl groups, so we concluded that more electron-donating groups could reduce the electron density of the A ring and make it difficult to form π-π bonds with amino acids containing aromatic groups.
In the case of constant A ring substituents, changes of substituents on the B ring can also affect the activities of these compounds. Contrary to the A ring, derivatives H17–H32 with a methoxyl at the para-position displayed better activities than at the meta-position (compounds H1–H16). These indicated that the position of the substituent had an influence on the antitumoral activity.
2.2.2. Kinase Inhibitory Activity
All these newly synthesized compounds were evaluated for their inhibitory activities against EGFR kinases using a solid-phase ELISA assay. The approved EGFR inhibitor drug erlotinib was used as a positive control. The inhibition constants (IC50) of the compounds were summarized in Table 3.
Table 3.
Inhibition activities (IC50, μM a) of all compounds against epidermal growth factor receptor (EGFR).
| Compounds | IC50, μM a | Compounds | IC50, μM a |
|---|---|---|---|
| H1 | 8.47 | H17 | 7.89 |
| H2 | 0.65 | H18 | 0.48 |
| H3 | 2.12 | H19 | 1.25 |
| H4 | 0.23 | H20 | 0.08 |
| H5 | 4.31 | H21 | 3.56 |
| H6 | 5.96 | H22 | 5.07 |
| H7 | 26.94 | H23 | 24.58 |
| H8 | 7.20 | H24 | 6.65 |
| H9 | 16.20 | H25 | 15.33 |
| H10 | 19.95 | H26 | 18.55 |
| H11 | 14.10 | H27 | 13.78 |
| H12 | 12.81 | H28 | 12.34 |
| H13 | 11.86 | H29 | 11.24 |
| H14 | 10.33 | H30 | 10.02 |
| H15 | 9.87 | H31 | 9.66 |
| H16 | 9.01 | H32 | 8.64 |
| Erlotinib | 0.03 |
a EGFR inhibitory activity was measured by the solid-phase ELISA assay. Values are the average of six independent experiments run in triplicate. Variation was generally 5%–10%.
The EGFR kinase inhibitory activity results of the tested compounds correlated with the structural relationships (SAR) of their inhibitory effects on the cell proliferation assay. Among the tested compounds, compound H20 showed potent anticancer activity with IC50 of 0.08 μM, which was comparable to the positive control erlotinib (IC50 = 0.03 μM). Other tested compounds displayed moderate inhibitory activities, with IC50 values ranging from 0.35 μM to 24.58 μM. This suggests that the potent inhibitory effects of the synthetic compounds on the cell proliferation assay were basically related to their kinase inhibitory activities.
2.2.3. Cytotoxicity Test
All the target compounds H1–H32 were evaluated for their toxicity against 293T human kidney epithelial cells at the median cytotoxic concentration (CC50) value of the tested compounds determined by the MTT assay. As displayed in Table 4, these compounds were tested at multiple doses to study the viability of 293T cells. Judging from the median cytotoxic concentration (CC50) data, all of the tested compounds demonstrated similar cytotoxic activities as erlotinib in vitro against human kidney epithelial 293T cell.
Table 4.
The median cytotoxic concentration (CC50, μM a) data of tested compounds
| Compounds | CC50, μM a | Compounds | CC50, μM a |
|---|---|---|---|
| H1 | 98.56 | H17 | 94.17 |
| H2 | 87.45 | H18 | 90.64 |
| H3 | 92.32 | H19 | 92.58 |
| H4 | 98.83 | H20 | 89.86 |
| H5 | 92.50 | H21 | 82.61 |
| H6 | 81.75 | H22 | 84.67 |
| H7 | 92.93 | H23 | 85.76 |
| H8 | 79.69 | H24 | 81.29 |
| H9 | 83.43 | H25 | 85.60 |
| H10 | 95.79 | H26 | 94.30 |
| H11 | 86.52 | H27 | 89.42 |
| H12 | 80.24 | H28 | 82.16 |
| H13 | 93.35 | H29 | 94.37 |
| H14 | 90.14 | H30 | 88.54 |
| H15 | 88.23 | H31 | 84.77 |
| H16 | 81.87 | H32 | 83.63 |
| Erlotinib | 89.32 |
a The cytotoxicity of each compound was expressed as the concentration of compound that reduced cell viability to 50% (CC50).
2.2.4. Analysis of Apoptosis
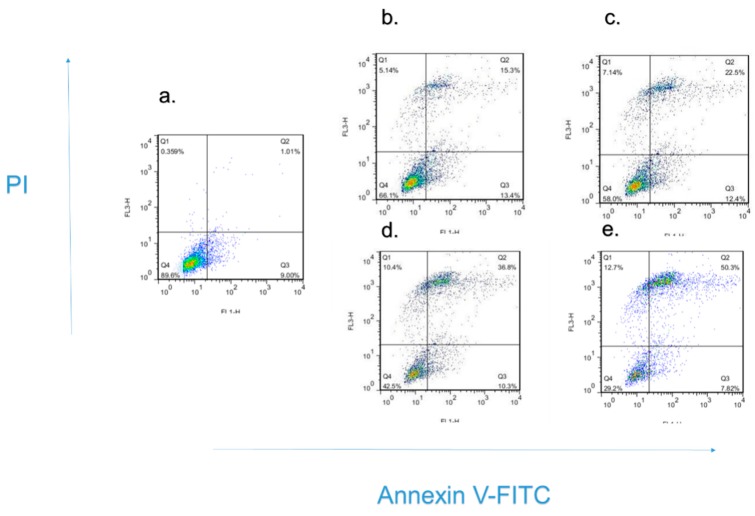
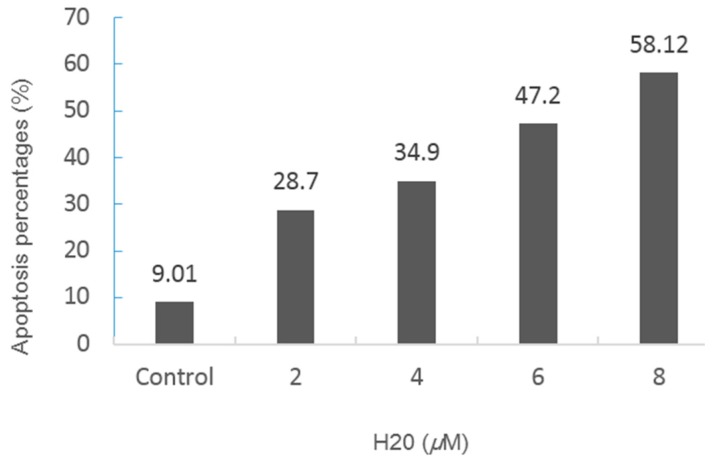
To verify whether the inhibition of cell growth of HeLa cell lines was related to cell apoptosis, we decided to use flow cytometry with an Annexin V-FITC/PI Apoptosis Detection Kit to induce the HeLa cell apoptosis with compound H20. The uptake of Annexin V-FITC/PI markedly increased, and the uptake of normal cells was significantly decreased in a time-dependent manner. According to the annotated data, the percentage of apoptotic cells was significantly elevated directly and in a dose-dependent manner. The results are shown in Figure 2 and Figure 3. As can be seen, the percentages of cell apoptosis were 9.01%, 28.7%, 34.9%, 47.7%, 58.12%, in response to 0, 2.0, 4.0, 6.0, 8.0 μM concentrations of compound H20, respectively. This leads to the conclusion that the percentage of apoptotic cells significantly increased after treatment with high doses of H20.
Figure 2.
Compound H20 induced apoptosis in Hela cells with the density of 0, 2.0, 4.0, 6.0, 8.0 μM (a–e). HeLa cells were treated with for 24 h. Values represent the mean ± S.D, n = 3, p < 0.05 versus control. The percentage of cells in each part was indicated.
Figure 3.
The percentage of early apoptotic cells and late apoptotic cells located in the upper right quadrant. Images were representative of three independent experiments. Values represent the mean ± S.D, n = 3, p < 0.05 versus control.
2.3. Molecular Docking
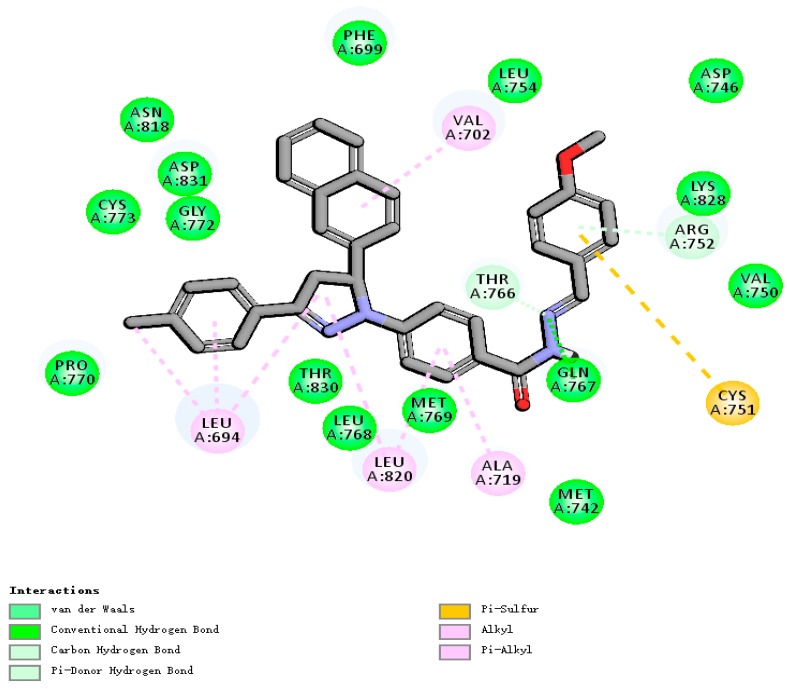
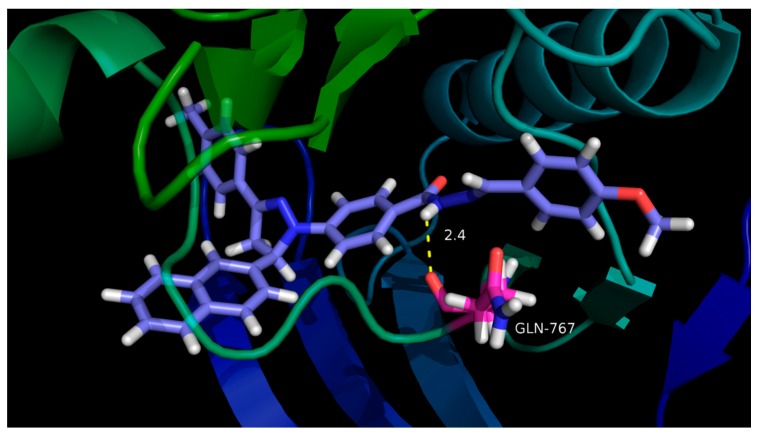
To gain better understanding of the potency of the 32 compounds and guide further SAR studies, we examined the interaction of these compounds with EGFR (PDB code: 1M17) by molecular docking. A simulation of binding between the compounds and the ATP binding sites in EGFR was performed. All docking runs used the DS 4.5 software (Discovery Studio 4.5, Accelrys, Co. Ltd., Beijing, China). The obtained results, presented in Figure 4 and Figure 5, show the optimal binding mode of compound H20 interacting with the 1M17 protein. The amino acid residues of EGFR which had interactions with compound H20 were labeled. In the proposed binding mode, compound H20 was nicely bound to the ATP binding pocket of EGFR through hydrogen bond interactions and hydrophobic interactions.
Figure 4.
Molecular docking 2D model of the interaction between compound H20 and EGFR enzyme: for clarity, only interacting residues are displayed.
Figure 5.
Molecular docking 3D modeling of compound H20 with the EGFR enzyme: for clarity, only interacting residues are displayed.
In the binding model, compound H20 was well bound to the EGFR protein with Gln767, Thr766, Arg752, Val702, Leu694, Leu820, Ala719, the seven amino acids located in the binding pocket of the protein, playing an important role in the combination with compound H20. As we can see from Figure 4 and Figure 5, Gln767 formed conventional hydrogen bonds and Thr766 formed Van der Waals bonds with the nitrogen atom of the Schiff base group, which enhanced the combination activity of compound H20. Moreover, a carbon–hydrogen bond and π-sulfur bonds were found between Arg828 and Cys751 with a benzene ring. Meanwhile the Leu694 formed three alkyl bonds with the methyl, benzene ring and dihydropyrazole ring. Alkyl and π-alkyl bonds were displayed in presence of Leu820 and dihydropyrazole ring and benzene ring, respectively. Moreover, Ala719 also formed π-alkyl bonds with the benzene ring. Naphthalene was also bonded with Val702 by a π-alkyl bond. In molecular docking 3D modeling, compound H20 was nicely bound to the ATP binding site through the hydrogen bond with the backbone of Gln767 (distance = 2.4 Å) which increased the binding affinity dramatically in theory, as displayed in Figure 5.
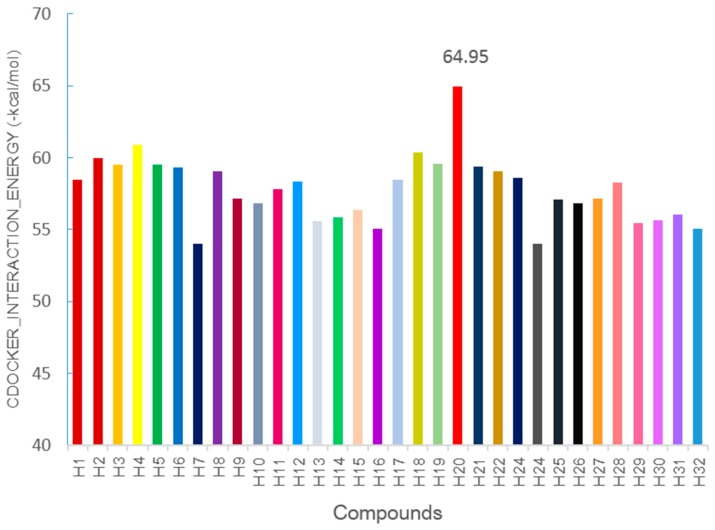
In addition, the predicted binding interaction energy was used as the criterion for ranking; the estimated interaction energies of other synthesized compounds ranged from −64.95 to −52.99 kcal/mol, as displayed in Figure 6 with a histogram. The selected compound of H20 had a best estimated binding free energy of −64.95 kcal/mol for EGFR. These molecular docking results, along with the biological assay data, suggested that compound H20 is a potential inhibitor of EGFR.
Figure 6.
The histogram about CDOCKER_INTERACTION_ENERGY (−kcal/mol) of compounds (H1–H32) for EGFR.
3. Experimental Section
3.1. General Information
All chemicals and reagents used in current study were commercially available analytical or chemically pure grade reagents, unless otherwise indicated. All the reactions were monitored by thin-layer chromatography (TLC) on glass-backed silica gel sheets (silica GF 254) and visualized using UV (254 or 365 nm). Column chromatography separations were performed on silica gel (200–300 mesh) using EtOAc/petroleum ether as eluents. Melting points (uncorrected) were determined using an X-4 MP apparatus (Taike Corp, Beijing, China). All the 1H-NMR and 13C-NMR spectra were recorded using a model DPX400 spectrometer (Bruker, Billerica, MA, USA) in DMSO-d6 and chemical shifts were reported in δ (ppm) units relative to the internal standard tetramethylsilane (TMS). Mass spectra (MS) were recorded using a Mariner System 5304 mass spectrometer (Isoprime, Manchester, UK).
3.2. General Synthesis Procedure of Benzohydrazide Derivatives Containing Dihydropyrazole Moieties
3.2.1. Synthesis of Methyl 4-Hydrazinylbenzoate (B)
4-Hydrazinylbenzoic acid (3.0 g, 0.020 mol) was suspended in anhydrous methanol (30 mL) and thionyl chloride (5 mL) was added dropwise at 0 °C over 30 min. Then the reaction mixture was stirred at room temperature for 20–24 h, the precipitate that formed was filtered, washed with petroleum ether and ethanol (5:1) three times to remove residual 4-hydrazinylbenzoic acid, and dried to give the title 4-hydrazinylbenzoate.
3.2.2. General Synthetic Procedure for Diverse Substituted Chalcones E1–E16
The diverse substituted chalcones E1–E16 were synthesized by reacting the appropriate substituted acetophenone (3.0 mmol) and one equivalent of naphthaldehyde (0.5 g, 3.0 mmol) using 40% potassium hydroxide (2 mL) as catalyst in ethanol (20 mL). The reaction mixture was stirred at room temperature for 6–8 h, the yellow precipitate that formed was filtered, washed with petroleum ether and ethanol (5:1) three times to remove impurities, and dried to give the diverse substituted chalcones.
3.2.3. General Synthetic Procedure for Diverse Substituted Chalcones F1–F16
Added acetic acid (1 mL) was used as an catalytic agent. The cyclization reaction of different substituted chalcones E1–E16 (3.0 mmol) and methyl 4-hydrazinylbenzoate (3.0 mmol) was performed in refluxing ethanol (20 mL) at 80 °C for 20–24 h to afford compounds F1–F16, as monitored by thin layer chromatography (TLC). The crude products were purified in good yields by recrystallization from ethanol, ethyl acetate and acetone (1:1:0.05).
3.2.4. General Synthetic Procedure for Compounds G1–G16
Compounds F1–F16 (3.0 mmol) were dissolved in DMF (10 mL) and excess hydrazine hydrate (80%, 5 mL) was added dropwise in methanol (20 mL). The solution was refluxed at 70 °C for 20–24 h, and then poured into water (50 mL) and extracted with ethyl acetate (3 × 30 mL). The organic layer was collected, and washed with brine. The organic layer was dried over anhydrous sodium sulfate and concentrated in vacuo to obtain yields 60%–80% of crude products G1–G16.
3.2.5. General Synthetic Procedure for the Title Compounds H1–H32
Equimolar amounts of compounds G1–G16 (2.0 mmol) and 3- or 4-methoxybenzaldehyde (2.0 mmol) were dissolved in ethanol (20 mL), then acetic acid (1 mL) was added as catalytic agent. The reaction mixture solution continued to stirred at room temperature for 6–8 h. The resulting solid was extracted with petroleum ether for column chromatography. Column chromatography was performed using silica gel (200–300 mesh), eluting with ethyl acetate and petroleum ether (1:2, v/v), to give the title products. All of the target compounds gave satisfactory analytical and 1H-NMR (in Supplementary Materials) and MS spectroscopic data, in accordance with their depicted structures.
(E)-N′-(3-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)benzo-hydrazide (H1). Yellow powder. Yield: 75.01%. m.p. 238–240 °C. 1H-NMR (DMSO-d6): 3.28–3.34 (dd, J1 = 5.44 Hz, J2 = 17.72 Hz, 1H); 3.79 (s, 3H); 4.04–4.11 (dd, J1 = 12.32 Hz, J2 = 17.56 Hz, 1H); 5.81–5.85 (dd, J1 = 5.40 Hz, J2 = 12.12 Hz, 1H); 6.97–6.99 (d, J = 7.96 Hz, 1H); 7.13–7.15 (d, J = 8.88 Hz, 2H); 7.23 (s, 1H); 7.32–7.36 (m, 1H); 7.38–7.53 (m, 6H); 7.73–7.75 (d, J = 8.80 Hz, 2H); 7.83–7.84 (m, 2H); 7.88–7.93 (m, 4H); 8.33 (s, 1H); 11.55 (s, 1H). 13C-NMR (DMSO-d6): 163.07, 161.08, 149.84, 139.88, 133.40, 132.34, 129.71, 129.66, 129.40, 129.21, 128.13, 128.08, 127.57, 126.97, 126.50, 125.07, 124.26, 123.28, 114.75, 112.49, 63.20, 60.40, 55.72, 43.22. MS m/z: [M + H]+ 525.6 (C34H28N4O2). Anal. Calcd. for C34H28N4O2: C, 78.34; H, 5.45; N, 10.38. Found: C, 77.84; H, 5.38; N, 10.68.
(E)-N′-(3-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-(o-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)benzo-hydrazide (H2).Yellow powder. Yield: 84.15%. m.p. 242–244 °C. 1H-NMR (DMSO-d6): 2.76 (s,3H); 3.34–3.39 (dd, J1 = 5.48 Hz, J2 = 11.92 Hz, 1H); 3.78 (s, 3H); 4.11–4.18 (dd, J1 = 12.12 Hz, J2 = 17.84 Hz, 1H); 5.74–5.79 (dd, J1 = 5.56 Hz, J2 = 12.36 Hz, 1H); 6.97–6.99 (d, J = 7.72 Hz, 1H); 7.09–7.11 (d, J = 8.80 Hz, 2H); 7.23–7.41 (m, 7H); 7.48–7.53 (m, 3H); 7.73–7.75 (d, J = 8.72 Hz, 2H); 7.87–7.92 (m, 4H); 8.32 (s, 1H); 11.54 (s, 1H). 13C-NMR (DMSO-d6): 163.12, 161.08, 148.84, 139.90, 133.46, 132.34, 129.71, 129.66, 129.50, 129.21, 128.13, 127.57, 126.97, 126.50, 125.07, 124.26, 123.28, 114.75, 112.49, 60.33, 55.73, 43.42. MS m/z: [M + H]+ 539.6 (C35H30N4O2). Anal. Calcd. for C35H30N4O2: C, 78.04; H, 5.61; N, 10.40. Found: C, 77.92; H, 5.56; N, 10.34.
(E)-N′-(3-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-(m-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)benzo-hydrazide (H3). Yellow powder. Yield: 87.67%. m.p. 232–234 °C. 1H-NMR (DMSO-d6): 2.37 (s, 3H); 3.26–3.32 (dd, J1 = 5.12 Hz, J2 = 17.64 Hz, 1H); 3.79 (s, 3H); 4.01–4.09 (dd, J1 = 12.16 Hz, J2 = 17.64 Hz, 1H); 4.37–4.40 (t, 1H); 5.80–5.84 (dd, J1 = 5.36 Hz, J2 = 12.16 Hz, 1H); 6.97–6.99 (d, J = 8.08 Hz, 1H); 7.13–7.15 (d, J = 8.68 Hz, 2H); 7.23–7.24 (d, J = 6.12 Hz, 2H); 7.33–7.39 (m, 3H); 7.49–7.51 (m, 2H); 7.60–7.62 (d, J = 7.72 Hz, 1H); 7.68 (s, 1H); 7.73–7.76 (d, J = 8.60 Hz, 2H); 7.86–7.93 (m, 4H); 8.33 (s, 1H); 11.55 (s, 1H). 13C-NMR (DMSO-d6): 163.07, 161.07, 149.84, 139.88, 133.40, 132.34, 129.71, 129.66, 129.40, 129.21, 128.13, 128.08, 127.57, 126.97, 126.50, 125.07, 124.26, 123.28, 114.75, 112.49, 60.23, 55.72, 43.35. MS m/z: [M + H]+ 539.6 (C35H30N4O2). Anal. Calcd. for C35H30N4O2: C, 78.04; H, 5.61; N, 10.40. Found: C, 77.94; H, 5.54; N, 10.35.
(E)-N′-(3-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)benzo-hydrazide (H4). Yellow powder. Yield: 87.18%. m.p. 233–235 °C. 1H-NMR (DMSO-d6): 2.35 (s, 3H); 3.25–3.30 (dd, J1 = 4.24 Hz, J2 = 17.60 Hz, 1H); 3.79 (s, 3H); 4.02–4.08 (dd, J1 = 12.52 Hz, J2 = 17.80 Hz, 1H); 5.78–5.82 (dd, J1 = 5.52 Hz, J2 = 12.24 Hz, 1H); 6.97–6.99 (d, J = 8.04 Hz, 1H); 7.11–7.13 (d, J = 8.80 Hz, 2H); 7.23–7.29 (m, 4H); 7.32–7.40 (m, 2H); 7.49–7.51 (m, 2H); 7.71–7.75 (m, J1 = 4.12 Hz, J2 = 4.88 Hz, 4H); 7.87–7.93 (m, 4H); 8.33 (s, 1H); 11.54 (s, 1H). 13C-NMR (DMSO-d6): 163.09, 161.05, 150.14, 138.88, 133.40, 129.71, 129.66, 129.40, 129.21, 128.13, 128.08, 127.57, 126.97, 126.50, 125.07, 124.26, 123.28, 114.75, 112.49, 60.54, 55.72, 43.35. ESI- MS m/z: [M + H]+ 539.6 (C35H30N4O2). Anal. Calcd. for C35H30N4O2: C, 78.04; H, 5.61; N, 10.40. Found: C, 77.95; H, 5.56; N, 10.36.
(E)-4-(3-(4-Ethylphenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene)-benzohydrazide (H5). Yellow powder. Yield: 76.02%. m.p. 241–243 °C. 1H-NMR (DMSO-d6): 3.25–3.30 (dd, J1 = 5.20 Hz, J2 = 17.64 Hz, 1H); 3.79–3.81 (d, J = 9.12 Hz, 6H); 4.00–4.08 (dd, J1 = 12.04 Hz, J2 = 17.52 Hz, 1H); 5.76–5.80 (dd, J1 = 5.16 Hz, J2 = 12.08 Hz, 1H); 6.97–7.04 (m, 3H); 7.09–7.11 (d, J = 8.76 Hz, 2H); 7.23 (s, 2H); 7.30–7.40 (m, 3H); 7.49–7.51 (m, 2H); 7.71–7.78 (m, 4H); 7.87–7.93 (m, 4H); 8.32 (s, 1H); 11.53 (s, 1H). 13C-NMR (DMSO-d6): 163.07, 161.06, 160.56, 149.95, 149.85, 146.85, 146.77, 145.72, 140.01, 139.94, 133.40, 132.88, 129.62, 129.39, 128.92, 128.61, 128.23, 128.07, 127.59, 126.95, 126.63, 126.57, 125.02, 124.91, 124.27, 122.85, 114.75, 114.67, 112.26, 60.22, 55.72, 43.57, 43.44. MS m/z: [M + H]+ 553.2 (C36H32N4O2). Anal. Calcd. for C36H32N4O2: C, 78.24; H, 5.84; N, 10.14. Found: C, 78.12; H, 5.79; N, 10.11.
(E)-N′-(3-Methoxybenzylidene)-4-(3-(4-methoxyphenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-benzohydrazide (H6). Yellow powder. Yield: 63.64%. m.p. 234–236 °C. 1H-NMR (DMSO-d6): 2.62–2.67 (dd, J1 = 7.52 Hz, J2 = 15.08 Hz, 2H); 3.25–3.31 (dd, J1 = 5.12Hz, J2 = 17.56 Hz, 1H); 3.79–3.84 (t, 3H); 4.00–4.08 (dd, J1 = 12.08 Hz, J2 = 17.92 Hz, 1H); 5.77–5.82 (dd, J1 = 5.36 Hz, J2 = 12.16 Hz, 1H); 6.97–6.99 (d, J = 7.84 Hz, 1H); 7.10–7.14 (m, 2H); 7.23 (s, 2H); 7.29–7.40 (m, 4H); 7.49–7.51 (m, 2H); 7.73–7.75 (d, J = 8.08 Hz, 4H); 7.87–7.92 (m, 5H); 8.33 (s, 1H); 11.54 (s, 1H). 13C-NMR (DMSO-d6): 163.07, 161.06, 149.96, 146.81, 146.72, 145.73, 139.95, 133.40, 132.88, 129.86, 129.64, 129.38, 128.92, 128.62, 128.24, 128.07, 127.58, 126.96, 126.64, 125.04, 124.26, 123.09, 114.75, 112.38, 63.08, 55.72, 43.57, 43.42. MS m/z: [M + H]+ 555.6 (C35H30N4O3). Anal. Calcd. for C35H30N4O3: C, 75.79; H, 5.45; N, 10.10. Found: C, 75.67; H, 5.43; N, 10.02.
(E)-N′-(3-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)benzohydrazide (H7). Yellow powder. Yield: 77.42%. m.p. 230–232 °C. 1H-NMR (DMSO-d6): 3.35–3.41 (dd, J1 = 5.68 Hz, J2 = 17.56 Hz, 1H); 3.70 (s, 3H); 3.79 (s, 3H); 3.86 (s, 6H); 4.01–4.05 (dd, J1 = 6.28 Hz, J2 = 15.16 Hz, 1H); 5.82–5.86 (dd, J1 = 5.12 Hz, J2 = 16.40 Hz, 1H); 6.97–6.99 (d, J = 8.04 Hz, 1H); 7.10–7.11 (m, 4H); 7.23 (s, 2H); 7.33–7.40 (m, 2H); 7.49–7.51 (m, 2H); 7.72–7.74 (d, J = 8.68 Hz, 2H); 7.85–7.94 (m, 4H); 8.33 (s, 1H); 11.53 (s, 1H). 13C-NMR (DMSO-d6): 163.92, 163.63, 162.97, 162.32, 162.23, 161.09, 160.70, 147.83, 146.98, 146.16, 139.53, 136.01, 135.94, 135.87, 133.37, 132.92, 129.70, 129.64, 129.37, 128.95, 128.08, 127.53, 127.13, 126.67, 125.14, 124.24, 123.98, 114.75, 112.91, 109.54, 109.36, 104.85, 104.68, 104.51, 63.60, 55.72, 42.98. MS m/z: [M + H]+ 615.7 (C37H34N4O5). Anal. Calcd. for C37H34N4O5: C, 72.30; H, 5.58; N, 9.11. Found: C, 72.18; H, 5.56; N, 9.08.
(E)-4-(3-(4-Ethoxyphenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene)-benzohydrazide (H8). Yellow powder. Yield: 82.31%. m.p. 233–235 °C. 1H-NMR (DMSO-d6): 1.32–1.36 (t, 3H); 3.23–3.29 (dd, J1 = 5.12 Hz, J2 = 17.56 Hz, 1H); 3.78 (s, 3H); 3.99–4.10 (m, 3H); 5.74–5.78 (dd, J1 = 5.52 Hz, J2 = 12.08 Hz, 1H); 6.96–7.02 (dd, J1 = 1.76 Hz, J2 = 11.40 Hz, 3H); 7.09–7.11 (d, J = 8.84 Hz, 2H); 7.23 (s, 2H); 7.32–7.40 (m, 2H); 7.47–7.52 (m, 2H); 7.73–7.77 (m, 4H); 7.86–7.92 (m, 4H); 8.33 (s, 1H); 11.53 (s, 1H). 13C-NMR (DMSO-d6): 163.01, 161.07, 159.98, 149.86, 146.85, 146.76, 140.01, 133.40, 132.87, 129.62, 129.38, 128.91, 128.43, 128.17, 128.07, 127.59, 126.95, 126.56, 125.02, 124.75, 124.27, 122.82, 115.08, 112.24, 63.69, 63.24, 55.72, 43.45. MS m/z: [M + H]+ 569.6 (C36H32N4O3). Anal. Calcd. for C36H32N4O3: C, 76.04; H, 5.67; N, 9.85. Found: C, 75.90; H, 5.65; N, 9.81.
(E)-4-(3-(2-Fluorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene)-benzohydrazide (H9). Yellow powder. Yield: 83.1%. m.p. 235–237 °C. 1H-NMR (DMSO-d6): 3.31–3.36 (dd, J1 = 5.52 Hz, J2 = 17.64 Hz, 1H); 3.79 (s, 3H); 4.11–4.19 (dd, J1 = 12.52 Hz, J2 = 18.84 Hz, 1H); 5.80–5.85 (dd, J1 = 5.12 Hz, J2 = 12.16 Hz, 1H); 6.97–6.99 (d, J = 7.68 Hz, 1H); 7.14–7.16 (d, J = 8.48 Hz, 2H); 7.23 (s, 2H); 7.29–7.33 (m, 3H); 7.40–7.52 (m, 4H); 7.74–7.76 (d, J = 8.44 Hz, 2H); 7.88–8.02 (m, 5H); 8.33 (s, 1H); 11.55 (s, 1H). 13C-NMR (DMSO-d6): 163.97, 163.08, 161.91, 161.07, 149.52, 146.87, 146.76, 139.32, 133.39, 132.90, 129.67, 129.40, 128.98, 128.97, 128.94, 128.75, 128.24, 128.07, 127.57, 126.61, 125.08, 124.24, 123.30, 116.16, 114.75, 112.49, 63.42, 60.43, 55.72, 43.42. MS m/z: [M + H]+ 543.6 (C34H27FN4O2). Anal. Calcd. for C34H27FN4O2: C, 75.26; H, 5.02; F, 3.50; N, 10.33. Found: C, 75.16; H, 5.01; F, 3.48; N, 10.30.
(E)-4-(3-(3-Fluorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene)-benzohydrazide (H10). Yellow powder. Yield: 74.96%. m.p. 241–243 °C.1H-NMR (DMSO-d6): 3.30–3.36 (dd, J1 = 5.28 Hz, J2 = 17.72 Hz, 1H); 3.79 (s, 3H); 4.02–4.10 (dd, J1 = 12.96 Hz, J2 = 18.32 Hz, 1H); 5.84–5.89 (dd, J1 = 5.36 Hz, J2 = 12.28 Hz, 1H); 6.97–6.99 (d, J = 7.68 Hz, 1H); 7.15–7.17 (d, J = 8.76 Hz, 2H); 7.24–7.28 (m, 3H); 7.33–7.40 (m, 2H); 7.49–7.51 (m, 3H); 7.63–7.67 (m, 2H); 7.73–7.76 (d, J = 8.72 Hz, 2H); 7.88–7.93 (m, 4H); 8.33 (s, 1H); 11.56 (s, 1H). 13C-NMR (DMSO-d6): 163.87, 163.08, 162.31, 161.07, 149.32, 146.87, 146.66, 139.82, 133.39, 132.90, 129.66, 129.40, 128.99, 128.97, 128.94, 128.77, 128.71, 128.24, 128.07, 127.57, 126.61, 125.08, 124.24, 123.30, 116.16, 114.74, 112.49, 63.42, 60.23, 55.72, 43.42.MS m/z: [M + H]+ 543.6 (C34H27FN4O2). Anal. Calcd. for C34H27FN4O2: C, 75.26; H, 5.02; N, 10.33. Found: C, 75.14; H, 5.00; N, 10.31.
(E)-4-(3-(4-Fluorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene)-benzohydrazide (H11). Yellow powder. Yield: 80.56%. m.p. 235–237 °C. 1H-NMR (DMSO-d6): 3.28–3.32 (dd, J1 = 5.16 Hz, J2 = 17.84 Hz, 1H); 3.79 (s, 3H); 4.03–4.10 (dd, J1 = 10.04 Hz, J2 = 17.64 Hz, 1H); 5.81–5.85 (dd, J1 = 6.40 Hz, J2 = 12.28 Hz, 1H); 6.97–6.99 (d, J = 7.68 Hz, 1H); 7.12–7.14 (d, J = 8.80 Hz, 2H); 7.23 (s, 2H); 7.29–7.34 (m, 3H); 7.38–7.40 (d, J = 9.88 Hz, 1H); 7.50–7.53 (m, 2H); 7.72–7.75 (d, J = 8.72 Hz, 2H); 7.86–7.93 (m, 6H); 8.32 (s, 1H); 11.54 (s, 1H). 13C-NMR (DMSO-d6): 163.87, 162.35, 161.07, 150.32, 146.87, 146.66, 139.82, 134.39, 132.90, 129.66, 129.40, 128.99, 128.97 128.94, 128.77, 128.71, 128.24, 128.07, 127.57, 126.61, 125.08, 124.24, 123.30, 116.16, 114.74, 112.49, 63.45, 60.33, 55.74, 43.45. MS m/z: [M + H]+ 543.6 (C34H27FN4O2). Anal. Calcd. for C34H27FN4O2: C, 75.26; H, 5.02; N, 10.33. Found: C, 75.15; H, 4.99; N, 10.29.
(E)-4-(3-(3,5-Difluorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene) benzohydrazide (H12). Yellow powder. Yield: 77.14%. m.p. 225–227 °C. 1H-NMR (DMSO-d6): 3.30–3.36 (dd, J1 = 5.44 Hz, J2 = 18.08 Hz, 1H); 3.79 (s, 3H); 4.00–4.07 (dd, J1 = 12.40 Hz, J2 = 17.80 Hz, 1H); 5.88–5.92 (dd, J1 = 5.60 Hz, J2 = 12.40 Hz, 1H); 6.97–6.99 (d, J = 8.04 Hz, 1H); 7.17–7.23 (m, 4H); 7.28–7.40 (m, 3H); 7.48–7.54 (m, 4H); 7.73–7.76 (d, J = 8.78 Hz, 2H); 7.88–7.94 (m, 4H); 8.33 (s, 1H); 11.57 (s, 1H). 13C-NMR (DMSO-d6): 163.08, 161.01, 153.43, 149.96, 146.85, 146.58, 133.93, 139.14, 133.41, 132.89, 129.66, 129.63, 129.37, 128.82, 128.22, 128.07, 127.86, 127.56, 126.98, 126.59, 124.92, 124.26, 123.18, 114.75, 112.44, 104.02, 63.15, 56.46, 55.72, 43.52. MS m/z: [M + H]+ 561.6 (C34H26F2N4O2). Anal. Calcd. for C34H26F2N4O2: C, 72.85; H, 4.67; N, 9.99. Found: C, 72.74; H, 4.66; N, 9.96.
(E)-4-(3-(3-Chlorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene)-benzohydrazide (H13). Yellow powder. Yield: 87.17%. m.p. 231–233 °C. 1H-NMR (DMSO-d6): 3.30–3.36 (dd, J1 = 5.44 Hz, J2 = 17.76 Hz, 1H); 3.79 (s, 3H); 4.01–4.08 (dd, J1 = 12.16 Hz, J2 = 15.84 Hz, 1H); 5.84–5.88 (dd, J1 = 5.52 Hz, J2 = 12.36 Hz, 1H); 6.97–6.99 (d, J = 8.08 Hz, 1H); 7.15–7.17 (d, J = 8.84 Hz, 2H); 7.23 (s, 2H); 7.33–7.44 (m, 3H); 7.49–7.51 (m, 2H) 7.60–7.62 (d, J = 8.84 Hz, 1H); 7.74–7.76 (d, J = 8.76 Hz, 2H); 7.80–7.82 (d, J = 7.88 Hz, 1H); 7.87–7.93 (m, 4H); 8.01 (s, 1H); 8.33 (s, 1H); 11.57 (s, 1H). 13C-NMR (DMSO-d6): 170.09, 163.07, 161.08, 149.72, 146.89, 146.43, 139.54, 134.12, 133.39, 132.90, 131.37, 129.67, 129.40, 129.25, 128.20, 128.08, 127.56, 126.98, 126.63, 125.11, 124.24, 123.51, 114.75, 112.60, 63.20, 60.32, 55.72, 43.42. MS m/z: [M + H]+ 560.0 (75%), [M + 2 + H]+ 562.0 (25%), (C34H27ClN4O2). Anal. Calcd. for C34H27ClN4O2: C, 73.05; H, 4.87; N, 10.02;. Found: C, 72.90; H, 4.85; N, 9.98.
(E)-4-(3-(4-Chlorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene)-benzohydrazide (H14). Yellow powder. Yield: 72.41%. m.p. 236–238 °C. 1H-NMR (DMSO-d6): 3.32–3.38 (dd, J1 = 5.40 Hz, J2 = 17.60 Hz, 1H); 3.79 (s, 3H); 4.02–4.09 (dd, J1 = 11.84 Hz, J2 = 15.40 Hz, 1H); 5.82–5.87 (dd, J1 = 6.96 Hz, J2 = 12.44 Hz, 1H); 6.97–6.99 (d, J = 7.68 Hz, 1H); 7.13–7.15 (d, J = 8.80 Hz, 2H); 7.23 (s, 2H); 7.36–7.40 (m, 2H); 7.49–7.54 (m, 4H); 7.73–7.75 (d, J = 9.08 Hz, 2H); 7.83–7.93 (m, 6H); 8.33 (s, 1H); 11.58 (s, 1H). 13C-NMR (DMSO-d6): 170.11, 163.08, 161.07, 148.79, 146.89, 146.47, 139.53, 134.11, 133.39, 132.90, 131.27, 129.67, 129.40, 129.25, 128.20, 128.08, 127.56, 126.98, 126.63, 125.11, 124.24, 123.51, 114.75, 112.60, 63.20, 60.42, 55.72, 43.42. MS m/z: [M + H]+ 560.0 (75%), [M + 2 + H]+ 562.0 (25%), (C34H27ClN4O2). Anal. Calcd. for C34H27ClN4O2: C, 73.05; H, 4.87; N, 10.02. Found: C, 72.91; H, 4.86; N, 9.99.
(E)-4-(3-(3,4-Dichlorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxy-benzylidene)benzohydrazide (H15). Yellow powder. Yield: 71.11%. m.p. 234–236 °C. 1H-NMR (DMSO-d6): 3.32–3.37 (dd, J1 = 5.44 Hz, J2 = 16.48 Hz, 1H); 3.79 (s, 3H); 4.01–4.09 (dd, J1 = 12.36 Hz, J2 = 17.72 Hz, 1H); 5.86–5.90 (dd, J1 = 5.72 Hz, J2 = 12.32 Hz, 1H); 6.97–6.99 (d, J = 7.96 Hz, 1H); 7.16–7.18 (d, J = 8.80 Hz, 2H); 7.23 (s, 2H); 7.33–7.40 (m, 2H); 7.50–7.52 (m, 2H); 7.71–7.75 (m, 3H); 7.80–7.83 (m, 1H); 7.87–7.93 (m, 4H); 8.01–8.02 (m, 1H); 8.32 (s, 1H); 11.56 (s, 1H). 13C-NMR (DMSO-d6): 163.07, 161.08, 149.79, 146.78, 146.47, 139.52, 134.12, 133.39, 132.87, 131.27, 129.67, 129.40, 129.25, 128.20, 128.08, 127.56, 126.98, 126.63, 125.11, 124.24, 123.51, 114.75, 112.60, 60.52, 55.72, 43.42. MS m/z: [M + H]+ 594.5 (75%), [M + 2 + H]+ 598.5 (25%), (C34H26Cl2N4O2). Anal. Calcd. for C34H26Cl2N4O2: C, 68.81; H, 4.42; N, 9.44; O, 5.39. Found: C, 68.69; H, 4.41; N, 9.41.
(E)-4-(3-(4-Bromophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(3-methoxybenzylidene)-benzohydrazide (H16). Yellow powder. Yield: 77.83%. m.p. 245–247 °C. 1H-NMR (DMSO-d6): 3.27–3.33 (dd, J1 = 5.40 Hz, J2 = 17.60 Hz, 1H); 3.79 (s, 3H); 4.02–4.09 (dd, J1 = 12.28 Hz, J2 = 17.68 Hz, 1H); 5.82–5.87 (dd, J1 = 5.64 Hz, J2 = 12.28 Hz, 1H); 6.97–6.99 (d, J = 7.80 Hz, 1H); 7.13–7.15 (d, J = 8.76 Hz, 2H); 7.23 (s, 2H); 7.32–7.40 (m, 2H); 7.49–7.51 (m, 2H); 7.65–7.67 (d, J = 8.52 Hz, 2H); 7.72–7.78 (m, 4H); 7.87–7.93 (m, 4H); 8.32 (s, 1H); 11.55 (s,1H). 13C-NMR (DMSO-d6): 163.07, 161.08, 148.56, 146.82, 146.47, 139.71, 133.38, 132.90, 131.60, 129.67, 129.40, 128.94, 128.43, 128.25, 127.55, 126.98, 126.63, 125.11, 124.23, 123.53, 122.83, 114.75, 112.61, 63.40, 55.73, 43.12. MS m/z: [M + H]+ 604.5 (50%), [M + 2 + H]+ 606.5 (50%), (C34H27BrN4O2). Anal. Calcd. for C34H27BrN4O2: C, 67.67; H, 4.51; N, 9.28. Found: C, 67.54; H, 4.50; N, 9.25.
(E)-N′-(4-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)benzo-hydrazide (H17). Orange powder. Yield: 75.36%. m.p. 234–236 °C. 1H-NMR (DMSO-d6): 3.27–3.33 (dd, J1 = 4.20 Hz, J2 = 17.68 Hz, 1H); 3.79 (s, 3H); 4.04–4.11 (dd, J1 = 10.44 Hz, J2 = 17.56 Hz, 1H); 5.80–5.85 (dd, J1 = 5.56 Hz, J2 = 12.24 Hz, 1H); 6.98–7.00 (d, J = 8.44 Hz, 2H); 7.12–7.14 (d, J = 8.80 Hz, 2H); 7.39–7.53 (m, 6H); 7.61–7.63 (d, J = 8.20 Hz, 2H); 7.72–7.74 (d, J = 8.68 Hz, 2H); 7.82–7.84 (d, J = 7.04 Hz, 2H); 7.88–7.93 (m, 4H); 8.29 (s,1H); 11.41 (s, 1H). 13C-NMR (DMSO-d6): 163.03, 161.01, 149.84, 139.88, 133.40, 132.34, 129.71, 129.66, 129.40, 129.21, 128.13, 128.08, 127.57, 126.97, 126.50, 125.07, 124.26, 123.28, 114.75, 112.49, 63.20, 60.23, 55.73, 43.35. MS m/z: [M + H]+ 525.6 (C34H28N4O2). Anal. Calcd. for C34H28N4O2: C, 77.84; H, 5.38; N, 10.68. Found: C, 76.69; H, 5.39; N, 10.42.
(E)-N′-(4-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-(o-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)benzo-hydrazide (H18). Yellow powder. Yield: 70.12%. m.p. 240–242 °C. 1H-NMR (DMSO-d6): 2.76 (s, 3H); 3.33–3.39 (dd, J1 = 5.44 Hz, J2 = 17.28 Hz, 1H); 3.79 (s, 3H); 4.10–4.18 (dd, J1 = 12.08 Hz, J2 = 17.44 Hz, 1H); 5.74–5.80 (dd, J1 = 5.44 Hz, J2 = 12.16 Hz, 1H); 6.98–7.00 (d, J = 8.40 Hz, 2H); 7.08–7.10 (d, J = 8.76 Hz, 2H); 7.25–7.32 (m, 2H); 7.36–7.41 (m, 2H); 7.48–7.53 (m, 3H); 7.61–7.63 (d, J = 8.12 Hz, 2H); 7.72–7.74 (d, J = 8.68 Hz, 2H); 7.87–7.93 (m, 4H); 8.29 (s, 1H); 11.39 (s, 1H). 13C-NMR (DMSO-d6): 163.08, 161.07, 149.88, 139.92, 133.45, 132.34, 129.73, 129.65, 129.41, 129.21, 128.13, 128.08, 127.57, 126.97, 126.50, 125.07, 124.26, 123.28, 114.75, 112.49, 63.22, 55.72, 43.42. MS m/z: [M + H]+ 539.6 (C35H30N4O2). Anal. Calcd. for C35H30N4O2: C, 78.04; H, 5.61; N, 10.40. Found: C, 77.91; H, 5.60; N, 10.36.
(E)-N′-(4-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-(m-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)benzo-hydrazide (H19). Yellow powder. Yield: 82.68%. m.p. 230–232 °C. 1H-NMR (DMSO-d6): 2.37 (s, 3H); 3.26–3.32 (dd, J1 = 5.24 Hz, J2 = 17.56 Hz, 1H); 3.79 (s, 3H); 4.01–4.09 (dd, J1 = 12.08 Hz, J2 = 15.72 Hz, 1H); 5.80–5.84 (dd, J1 = 5.48 Hz, J2 = 12.32 Hz, 1H); 6.98–7.00 (d, J = 8.56 Hz, 2H); 7.12–7.14 (d, J = 8.84 Hz, 2H); 7.23–7.25 (d, J = 7.64 Hz, 1H); 7.33–7.40 (m, 2H); 7.49–7.51 (t, 2H); 7.60–7.63 (dd, J1 = 3.44 Hz, J2 = 4.32 Hz, 3H); 7.68 (s, 1H); 7.71–7.74 (d, J = 8.64 Hz, 2H); 7.86–7.93 (m, 4H); 8.29 (s, 1H); 11.40 (s, 1H). 13C-NMR (DMSO-d6): 163.03, 161.01, 149.84, 139.88, 133.40, 132.34, 129.71, 129.66, 129.40, 129.21, 128.13, 128.08, 127.57, 126.97, 126.50, 125.07, 124.26, 123.28, 114.75, 112.49, 63.20, 55.73, 43.35. MS m/z: [M + H]+ 539.6 (C35H30N4O2). Anal. Calcd. for C35H30N4O2: C, 78.04; H, 5.61; N, 10.40. Found: C, 77.66; H, 6.71; N, 9.46.
(E)-N′-(4-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)benzo-hydrazide (H20). Yellow powder. Yield: 73.91%. m.p. 241–243 °C. 1H-NMR (DMSO-d6): 2.35 (s, 3H); 3.23–3.29 (dd, J1 = 4.24 Hz, J2 = 17.64 Hz, 1H); 3.79 (s, 3H); 4.00–4.07 (dd, J1 = 12.20 Hz, J2 = 17.64 Hz, 1H); 5.76–5.81 (dd, J1 = 5.56 Hz, J2 = 12.16 Hz, 1H); 6.98–7.00 (d, J = 8.48 Hz, 2H); 7.10–7.13 (d, J = 8.72 Hz, 2H); 7.26–7.28 (d, J = 8.04 Hz, 2H); 7.37–7.40 (d, J = 8.40 Hz, 1H); 7.49–7.51 (m, 2H); 7.61–7.63 (d, J = 8.12 Hz, 2H); 7.71–7.73 (d, J = 8.04 Hz, 4H); 7.87–7.92 (m, 4H); 8.30 (s, 1H); 11.39 (s, 1H). 13C-NMR (DMSO-d6): 163.09, 161.05, 150.14, 138.58, 133.40, 129.71, 129.66, 129.40, 129.21, 128.13, 128.08, 127.57, 126.97, 126.50, 125.07, 124.26, 123.28, 114.75, 112.49, 60.54, 55.72, 43.35. MS m/z: [M + H]+ 539.6 (C35H30N4O2). Anal. Calcd. for C35H30N4O2: C, 78.04; H, 5.61; N, 10.40. Found: C, 77.60; H, 5.43; N, 10.36.
(E)-4-(3-(4-Ethylphenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(4-methoxybenzylidene)-benzohydrazide (H21). Orange powder. Yield: 76.19%. m.p. 239–241 °C. 1H-NMR (DMSO-d6): 3.24–3.29 (dd, J1 = 4.40 Hz, J2 = 17.36 Hz, 2H); 3.79–3.81 (d, J = 2.84 Hz, 6H); 3.99–4.07 (dd, J1 = 11.36 Hz, J2 = 17.80 Hz, 1H); 5.74–5.78 (dd, J1 = 5.20 Hz, J2 = 11.60 Hz, 1H); 6.98–7.03 (m, 4H); 7.09–7.11 (d, J = 8.52 Hz, 2H); 7.38–7.40 (d, J = 8.52 Hz, 1H); 7.49–7.51 (m, 2H); 7.61–7.63 (d, J = 7.88 Hz, 2H); 7.71–7.78 (m, 4H); 7.86–7.93 (m, 5H); 8.29 (s, 1H); 11.40 (s, 1H). 13C-NMR (DMSO-d6): 163.07, 161.06, 160.70, 149.95, 149.85, 146.85, 146.77, 145.72, 140.01, 139.94, 133.40, 132.88, 129.62, 129.39, 128.92, 128.61, 128.23, 128.07, 127.59, 126.95, 126.63, 126.57, 125.02, 124.91, 124.27, 122.85, 114.75, 114.67, 112.26, 63.06, 55.76, 43.57, 43.44. MS m/z: [M + H]+ 553.2 (C36H32N4O2). Anal. Calcd. for C36H32N4O2: C, 78.24; H, 5.84; N, 10.14. Found: C, 74.84; H, 5.53; N, 9.85.
(E)-N′-(4-Methoxybenzylidene)-4-(3-(4-methoxyphenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-benzohydrazide (H22). Yellow powder. Yield: 69.13%. m.p. 243–245 °C. 1H-NMR (DMSO-d6): 2.62–2.67 (dd, J1 = 7.56 Hz, J2 = 15.16 Hz, 2H); 3.25–3.30 (dd, J1 = 5.40 Hz, J2 = 7.64 Hz, 1H); 3.79–3.83 (t, 3H); 4.01–4.08 (dd, J1 = 12.36 Hz, J2 = 17.72 Hz, 1H); 5.77–5.82 (dd, J1 = 5.48 Hz, J2 = 12.12 Hz, 1H); 6.98–7.00 (d, J = 8.52 Hz, 2H); 7.10–7.13 (d, J = 8.80 Hz,2H); 7.29–7.31 (d, J = 8.20 Hz, 2H); 7.38–7.40 (d, J = 8.48 Hz, 1H); 7.49–7.51 (m, 2H); 7.61–7.63 (d, J = 8.28 Hz, 2H); 7.71–7.75 (m, 4H); 7.87–7.92 (m, 5H); 8.29 (s, 1H); 11.40 (s, 1H). 13C- NMR (DMSO-d6): 163.05, 161.06, 149.96, 146.81, 146.72, 145.73, 139.95, 133.40, 132.88, 129.86, 129.64, 129.38, 128.92, 128.62, 128.24, 128.07, 127.58, 126.96, 126.64, 125.04, 124.26, 123.09, 114.75, 112.38, 63.08, 55.77, 55.72, 43.57, 43.44. MS m/z: [M + H]+ 555.6 (C35H30N4O3). Anal. Calcd. for C35H30N4O3: C, 75.79; H, 5.45; N, 10.10. Found: C, 74.90; H, 5.99; N, 9.31.
(E)-N′-(4-Methoxybenzylidene)-4-(5-(naphthalen-2-yl)-3-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)benzohydrazide (H23). Yellow powder. Yield: 62.58%. m.p. 237–239 °C. 1H-NMR (DMSO-d6): 3.35–3.40 (dd, J1 = 5.64 Hz, J2 = 17.56 Hz, 1H); 3.70 (s,3H); 3.79 (s,3H); 3.86 (s, 6H); 4.01–4.08 (dd, J1 = 12.60 Hz, J2 = 17.56 Hz, 1H); 5.80–5.85 (dd, J1 = 5.20 Hz, J2 = 12.12 Hz, 1H); 6.98–7.00 (d, J = 8.60 Hz, 2H); 7.10–7.14 (m, 4H); 7.38–7.40 (d, J = 8.52 Hz, 1H); 7.49–7.51 (m, 2H); 7.61–7.63 (d, J = 8.20 Hz, 2H); 7.70–7.72 (d, J = 8.76 Hz, 2H); 7.85–7.94 (m, 4H); 8.29 (s, 1H); 11.39 (s, 1H). 13C-NMR (DMSO-d6): 163.95, 163.66, 162.97, 162.32, 162.23, 161.09, 160.70, 147.83, 146.98, 146.16, 139.53, 136.01, 135.94, 135.87, 133.37, 132.92, 129.70, 129.64, 129.37, 128.95, 128.08, 127.53, 127.00, 126.67, 125.14, 124.24, 123.98, 114.75, 112.91, 109.54, 109.36, 104.85, 104.68, 104.51, 63.65, 55.72, 42.98. MS m/z: [M + H]+ 615.7 (C37H34N4O5). Anal. Calcd. for C37H34N4O5: C, 72.30; H, 5.58; N, 9.11. Found: C, 71.41; H, 6.06; N, 8.31.
(E)-4-(3-(4-Ethoxyphenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(4-methoxybenzylidene)-benzohydrazide (H24). Yellow powder. Yield: 76.15%. m.p. 237–239 °C. 1H-NMR (DMSO-d6): 1.32–1.36 (t, 3H); 3.23–3.29 (dd, J1 = 5.16 Hz, J2 = 17.60 Hz, 1H); 3.79 (s, 3H); 3.99–4.10 (m,3H); 5.74–5.79 (dd, J1 = 5.56 Hz, J2 = 12.12 Hz, 1H); 6.98–7.02 (m, 4H); 7.08–7.10 (d, J = 8.84 Hz, 2H); 7.37–7.40 (m, 1H); 7.49–7.51 (m, 2H); 7.61–7.63 (d, J = 8.24 Hz, 2H); 7.70–7.77 (m, 4H); 7.86–7.9 2(m, 4H); 8.29 (s ,1H); 11.39 (s, 1H). 13C- NMR (DMSO-d6): 163.07, 161.05, 159.98, 149.86, 146.85, 146.76, 140.01, 133.40, 132.87, 129.62, 129.38, 128.91, 128.23, 128.17, 128.07, 127.59, 126.95, 126.56, 125.02, 124.75, 124.27, 122.82, 115.08, 112.24, 63.69, 63.04, 55.72, 43.55. MS m/z: [M + H]+ 569.6 (C36H32N4O3). Anal. Calcd. for C36H32N4O3: C, 76.04; H, 5.67; N, 9.85. Found: C, 75.04; H, 5.71; N, 9.58.
(E)-4-(3-(2-Fluorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(4-methoxybenzylidene)-benzohydrazide (H25). Yellow powder. Yield: 85.32%. m.p. 244–246 °C. 1H-NMR (DMSO-d6): 3.29–3.34 (dd, J1 = 5.52 Hz, J2 = 17.64 Hz, 1H); 3.79 (s, 3H); 4.11–4.18 (dd, J1 = 12.20 Hz, J2 = 17.68 Hz, 1H); 5.80–5.84 (dd, J1 = 5.60 Hz, J2 = 12.32 Hz, 1H); 6.99–7.01 (d, J = 8.16 Hz, 2H); 7.13–7.15 (d, J = 8.48 Hz, 2H); 7.28–7.33 (m, 2H); 7.40–7.52 (m, 5H); 7.61–7.63 (d, J = 7.92 Hz, 2H); 7.73–7.75 (d, J = 8.40 Hz,2H); 7.88–7.94 (m, 3H); 7.98–8.02 (m, 1H); 8.30 (s, 1H); 11.42 (s, 1H). 13C-NMR (DMSO-d6): 163.53, 162.31, 161.08, 149.22, 146.85, 146.76, 139.62, 133.42, 132.88, 129.66, 129.40, 128.99 128.97, 128.94, 128.77, 128.71, 128.24, 128.07, 127.57, 126.61, 125.08, 124.24, 123.30, 116.16, 114.74, 112.49, 63.23, 60.43, 55.73, 43.42. MS m/z: [M + H]+ 543.6 (C34H27FN4O2). Anal. Calcd. for C34H27FN4O2: C, 75.26; H, 5.02; N, 10.33. Found: C, 75.13; H, 5.01; N, 10.30.
(E)-4-(3-(3-Fluorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(4-methoxybenzylidene)-benzohydrazide (H26). Yellow powder. Yield: 75.6%. m.p. 240–242 °C. 1H-NMR (DMSO-d6): 3.29–3.35 (dd, J1 = 5.56 Hz, J2 = 17.80 Hz, 1H); 3.79 (s, 3H); 4.02–4.09 (dd, J1 = 12.28 Hz, J2 = 17.72 Hz, 1H); 5.84–5.88 (dd, J1 = 5.64 Hz, J2 = 12.36 Hz, 1H); 6.99–7.01 (d, J = 8.48 Hz, 2H); 7.14–7.17 (d, J = 8.84 Hz, 2H); 7.24–7.28 (m, 1H); 7.38–7.40 (d, J = 8.52 Hz, 1H ); 7.48–7.54 (m, 3H); 7.61–7.67 (m, 4H); 7.72–7.74 (d, J = 8.68 Hz, 2H); 7.88–7.93 (m, 4H); 8.29 (s, 1H); 11.42 (s, 1H). 13C-NMR (DMSO-d6): 163.08, 162.32, 161.09, 149.11, 146.82, 146.64, 139.82, 133.32, 132.90, 129.65, 129.40, 128.99, 128.97, 128.95, 128.76, 128.72, 128.24, 128.07, 127.57, 126.65, 125.02, 124.24, 123.30, 116.16, 114.72, 112.45, 63.36, 60.24, 55.72, 43.42. MS m/z: [M + H]+ 543.6 (C34H27FN4O2). Anal. Calcd. for C34H27FN4O2: C, 75.26; H, 5.02; N, 10.33. Found: C, 75.14; H, 5.00; N, 10.31.
(E)-4-(3-(4-Fluorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N'-(4-methoxybenzylidene)-benzohydrazide (H27). Yellow powder. Yield: 62.50%. m.p. 236–238 °C. 1H-NMR (400 MHz, DMSO-d6): 3.28–3.34 (dd, J1 = 5.52 Hz, J2 = 17.72 Hz, 1H); 3.79 (s, 3H); 4.02–4.09 (dd, J1 = 7.08 Hz, J2 = 17.80 Hz,1H); 5.80–5.84 (dd, J1 = 5.68 Hz, J2 = 12.28 Hz, 1H); 6.99–7.01 (d, J = 8.48 Hz, 2H); 7.11–7.13 (d, J = 8.80 Hz, 2H); 7.29–7.33 (t,2H); 7.38–7.40 (m, 1H); 7.49–7.51 (m, 2H); 7.61–7.63 (d, J = 8.28 Hz, 2H); 7.72–7.74 (d, J = 8.68 Hz, 2H); 7.86–7.93 (m, 6H); 8.29 (s, 1H); 11.41 (s, 1H). 13C-NMR (100 MHz, DMSO-d6): 163.93, 163.06, 162.29, 161.07, 149.00, 146.87, 146.66, 139.82, 133.39, 132.90, 129.66, 129.40, 128.99, 128.97, 128.94, 128.94, 128.77, 128.71, 128.24, 128.07, 127.57, 126.61, 125.08, 124.24, 123.30, 116.16, 114.74, 112.49, 63.33, 60.23, 55.71, 43.42. MS m/z: [M + H]+ 543.6 (C34H27FN4O2). Anal. Calcd. for C34H27FN4O2: C, 75.26; H, 5.02; N, 10.33. Found: C, 73.87; H, 4.97; N, 10.06.
(E)-4-(3-(3,5-Difluorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(4-methoxy-benzylidene)benzohydrazide (H28). Yellow powder. Yield: 69.3%. m.p. 238–240 °C. 1H-NMR (400 MHz, DMSO-d6): 3.30–3.36 (dd, J1 = 5.52 Hz, J2 = 17.88 Hz, 1H); 3.79 (s, 3H); 3.99–4.07 (dd, J1 = 12.36 Hz, J2 = 17.84 Hz, 1H); 5.87–5.92 (dd, J1 = 5.64 Hz, J2 = 12.44 Hz, 1H); 6.99–7.01 (d, J = 8.40 Hz, 2H); 7.17–7.19 (d, J = 8.80 Hz, 2H); 7.29–7.33 (t, 1H); 7.38–7.40 (d, J = 8.48 Hz, 1H); 7.48–7.54 (m, 4H); 7.61–7.63 (d, J = 8.28 Hz, 2H); 7.72–7.74 (d, J = 8.68 Hz, 2H); 7.88–7.94 (m, 4H); 8.29 (s, 1H); 11.43 (s, 1H). 13C-NMR (100 MHz, DMSO-d6): 163.09, 161.07, 153.53, 149.96, 146.85, 146.58, 133.93, 139.14, 133.41, 132.89, 129.66, 129.63, 129.37, 128.92, 128.22, 128.07, 127.86, 127.56, 126.98, 126.59, 124.92, 124.26, 123.18, 114.75, 112.44, 104.02, 63.17, 56.46, 55.73, 43.51. MS m/z: [M + H]+ 561.6 (C34H26F2N4O2). Anal. Calcd. for C34H26F2N4O2: C, 72.85; H, 4.67; N, 9.99;. Found: C, 70.13; H, 4.92; N, 9.59.
(E)-4-(3-(3-Chlorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(4-methoxybenzylidene)-benzohydrazide (H29). Orange powder. Yield: 87.21%. m.p. 241–243 °C. 1H-NMR (400 MHz, DMSO-d6): 3.29–3.34 (dd, J1 = 5.48 Hz, J2 = 17.64 Hz, 1H); 3.79 (s, 3H); 4.01–4.08 (dd, J1 = 12.16 Hz, J2 = 17.48 Hz, 1H); 5.84–5.88 (dd, J1 = 5.52 Hz, J2 = 12.32 Hz, 1H); 6.98–7.00 (d, J = 8.48 Hz, 2H); 7.14–7.16 (d, J = 8.88 Hz, 2H); 7.38–7.44 (m, 2H); 7.49–7.51 (m, 2H); 7.60–7.62 (d, J = 7.12 Hz, 3H); 7.72–7.74 (d, J = 8.76 Hz, 2H); 7.80–7.82 (d, J = 7.80 Hz, 1H); 7.87–7.93 (m, 4H); 8.01 (s, 1H); 8.29 (s, 1H); 11.41 (s, 1H). 13C-NMR (100 MHz, DMSO-d6): 170.21, 163.07, 161.09, 149.72, 14690, 146.43, 139.52, 134.32, 133.32, 132.90, 131.37, 129.67, 129.40, 129.35, 128.28, 128.08, 127.56, 126.98, 126.66, 125.11, 124.24, 123.51, 114.75, 112.60, 63.20, 55.72, 43.42. MS m/z: [M + H]+ 560.0 (75%), [M + 2 + H]+ 562.0 (25%), (C34H27ClN4O2). Anal. Calcd. for C34H27ClN4O2: C, 73.05; H, 4.87; N, 10.02. Found: C, 72.91; H, 4.86; N, 9.99.
(E)-4-(3-(4-Chlorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(4-methoxybenzylidene)-benzohydrazide (H30). Orange powder. Yield: 66.67%. m.p. 237–239 °C. 1H-NMR (400 MHz, DMSO-d6): 3.27–3.32 (dd, J1 = 5.44 Hz, J2 = 17.72 Hz, 1H); 3.79 (s, 3H); 4.01–4.06 (dd, J1 = 5.92 Hz, J2 = 17.48 Hz, 1H); 5.81–5.86 (dd, J1 = 5.72 Hz, J2 = 12.32 Hz, 1H); 6.98–7.00 (d, J = 8.48 Hz, 2H); 7.13–7.15 (d, J = 8.80 Hz, 2H); 7.38–7.40 (m, 1H); 7.49–7.54 (m, J1 = 4.00 Hz, J2 = 8.64 Hz, 4H); 7.61–7.63 (d, J = 8.28 Hz, 2H); 7.72–7.74 (d, J = 8.68 Hz, 2H); 7.83–7.93 (m, 6H); 8.30 (s, 1H); 11.42 (s, 1H). 13C-NMR (100 MHz, DMSO-d6): 170.81, 163.01, 161.08, 148.79, 146.89, 146.47, 139.73, 134.11, 133.39, 132.90, 131.27, 129.67, 129.40, 129.25, 128.20, 128.08, 127.56, 126.98, 126.63, 125.11, 124.24, 123.51, 114.75, 112.60, 63.40, 60.22, 55.72, 43.19. MS m/z: [M + H]+ 560.0 (75%), [M + 2 + H]+ 562.0 (25%), (C34H27ClN4O2). Anal. Calcd. for C34H27ClN4O2: C, 73.05; H, 4.87; N, 10.02. Found: C, 72.00; H, 4.95; N, 9.71.
(E)-4-(3-(3,4-Dichlorophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N'-(4-methoxy-benzylidene)benzohydrazide (H31). Orange powder. Yield: 73.45%. m.p. 243–245 °C. 1H NMR (400 MHz, DMSO-d6): 3.32–3.37 (dd, J1 = 5.44 Hz, J2 = 17.92 Hz, 1H); 3.79 (s, 3H); 4.01–4.08 (dd, J1 = 12.60 Hz, J2 = 17.72 Hz, 1H); 5.85–5.90 (dd, J1 = 5.80 Hz, J2 = 12.36 Hz, 1H); 6.98–7.00 (d, J = 8.48 Hz, 2H); 7.15–7.17 (d, J = 8.80 Hz, 2H); 7.38–7.40 (d, J = 8.48 Hz, 1H); 7.49–7.51 (m, 2H); 7.61–7.63 (d, J = 8.20 Hz, 2H); 7.71–7.74 (m, 3H); 7.79–7.82 (m, 1H); 7.87–7.93 (m, 4H); 8.02 (s, 1H); 8.29 (s, 1H); 11.42 (s, 1H). 13C NMR (100 MHz, DMSO-d6): 163.08, 161.07, 149.89, 146.78, 146.47, 139.52, 134.32, 133.39, 132.87, 131.27, 129.67, 129.40, 129.25, 128.20, 128.08, 127.56, 126.98, 126.33, 125.11, 124.24, 123.51, 114.75, 112.60, 60.52, 55.72, 43.42. MS m/z: [M + H]+ 594.5 (75%), [M + 2 + H]+ 598.5 (25%), (C34H26Cl2N4O2). Anal. Calcd. for C34H26Cl2N4O2: C, 68.81; H, 4.42; N, 9.44. Found: C, 68.68; H, 4.40; N, 9.42.
(E)-4-(3-(4-Bromophenyl)-5-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-N′-(4-methoxybenzylidene)-benzohydrazide (H32). Yellow powder. Yield: 70.90%. m.p. 238–240 °C. 1H-NMR (400 MHz, DMSO-d6): 3.26–3.32 (dd, J1 = 5.44 Hz, J2 = 17.80 Hz, 1H); 3.79 (s, 3H); 4.01–4.08 (dd, J1 = 11.96 Hz, J2 = 17.84 Hz, 1H); 5.81–5.85 (dd, J1 = 5.60 Hz, J2 = 12.20 Hz, 1H); 6.98–7.00 (d, J = 8.00 Hz, 2H); 7.13–7.15 (d, J = 8.48 Hz, 2H); 7.38–7.40 (d, J = 8.32 Hz, 1H); 7.49–7.51 (m, 2H); 7.61–7.67 (m, 4H); 7.72–7.77 (m, 4H); 7.87–7.93 (m, 4H); 8.29 (s,1H); 11.41 (s, 1H). 13C-NMR (100 MHz, DMSO-d6): 163.00, 161.08, 148.86, 146.89, 146.47, 139.71, 133.38, 132.90, 131.60, 129.67, 129.40, 128.94, 128.43, 128.25, 127.55, 126.98, 126.63, 125.11, 124.23, 123.53, 122.83, 114.75, 112.61, 63.40, 55.73, 43.12. MS m/z: [M + H]+ 604.5 (50%), [M + 2 + H]+ 606.5 (50%), (C34H27BrN4O2). Anal. Calcd. for C34H27BrN4O2: C, 67.67; H, 4.51; N, 9.28. Found: C, 66.49; H, 6.35; N, 7.86.
3.3. Biological Assays
3.3.1. Antiproliferation Activity
IC50 values of the test compounds against A549, MCF-7, Hela and HepG2 cells were obtained from State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, and were determined by a standard (MTT)-based colorimetric assay (Beyotime Inst. Biotech., Nanjing, China). Tumor cell lines were grown to mid-log phase and then diluted to 5 × 103–1 × 104 cells/mL making sure they were seeded in 96-well plates (Philas, Nanjing, China) at a density of 5 × 103–1 × 104 cells per well. The outer wells containing 1 × PBS solution of the plate are not used for the assay to avoid the possibility of edge effects. The plate was incubated at 37 °C in a 5% CO2 incubator in DMEM containing 10% fetal bovine serum (FBS, Gibco, Waltham, MA, USA) for 24 h. Then, we added a 100-μL series concentration of drug-containing medium into the wells to maintain the final concentration of drug as 100, 10, 1 and 0.1 μmol/L. After 12 h, cell survival was determined by the addition of a MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl trtrazolium bromide) solution (10 μL of 5 mg/mL MTT in 1 × PBS). We waited for 4 h proliferation, discarded the medium and quickly added 150 μL DMSO per well, shaking the plates well for 10 min to ensure complete dissolution. Optical absorbance was measured at 570 nm on a microplate reader (Bio-Rad, Hercules, CA, USA). Survival ratios are expressed in percentages with respect to untreated cells. The experiments were replicated at least three times to verify the methodology reproducibility when using the above-mentioned conditions. The results were summarized in Table 2.
3.3.2. Kinase Assay
EGFR tyrosine kinase activity was determined by an enzyme linked-immunosorbent assay (ELISA) in 96-well plates precoated in a 10 mL reaction volume including 4 mL diluted test compounds, 4 mL substrate and 2 mL ATP. After incubation for 1 h at 37 °C, the reactions were stopped with 10 mL 2% (v/v) H3PO4. The plates were then aspirated and washed twice with 200 mL 0.9% (w/v) NaCl, and incorporation of Pi was determined with an LX300 Epson Diagnostic microplate reader. The residual kinase activities for each concentration of compound and the compound IC50 values were expressed in percentages with respect to untreated cells. The experiments were replicated at least three times to verify the methodology reproducibility when using the above-mentioned conditions. The results were summarized in Table 3.
3.3.3. Cytotoxicity Assay
The cytotoxic activity in vitro was measured by the colorimetric MTT assay. Cells were incubated in a 96-well plate at a density of 105 cells per well with various concentrations of compounds (160, 40, 10, 2.5, 0.25 μM) in distilled 10% DMSO (10 μL) for 24 h. For the cytotoxicity assay, 20 μL of MTT (5 mg/mL) was added per well 4 h before the end of the incubation. After removing the supernatant, 200 μL DMSO was added to dissolve the formazan crystals. The absorbance at λ 570 nm was read on an LX300 Epson Diagnostic microplate reader, untreated cells were used as negative controls. The results are summarized in Table 4.
3.3.4. Cell Apoptosis Assay by Flow Cytometry
For Annexin V/PI assays, HeLa cells were stained with Annexin VFITC and PI and then monitored for apoptosis by flow cytometry. Briefly, 5 × 103 cells were seeded in 6-well plates for 24 h and then were treated with H20 (2.0, 4.0, 6.0, 8.0 μM) for 24 h. Then, cells were collected and washed twice with PBS and stained with 5 μL Annexin V-FITC and 5 μL PI (5 μg/mL) in 1× binding buffer (10 mM HEPES, pH 7.4, 140 mM NaOH, 2.5 mM CaCl2) for 15 min at room temperature in the dark. Apoptotic cells were quantified using a BD Accuri C6 Flow Cytometer (Becton, Dickinson and Company, Clifton, NJ, USA). Statistical analysis was done using Flowjo 7.6.1 software. Both early apoptotic (AnnexinV-positive, PI-negative) and late apoptotic (double positive of Annexin V and PI) cells were detected.
3.4. Docking Simulations
The crystal structures of the proteins complex were retrieved from the RCSB Protein Data Bank (PDB code: 1M17). Then, three-dimensional structures of the aforementioned compounds were constructed using Chem 3D Ultra 14.0 software (Cambridge Soft Corporation, Boston MA, USA), then they were energetically minimized by using MMFF94 with 5000 iterations and minimum RMS gradient of 0.10. Molecular docking of compound H20 into the three-dimensional EGFR complex structure was carried out using the Discovery Studio (Discovery Studio 4.5, Accelrys, Co. Ltd., Beijing, China) software as implemented through the graphical user interface DS-CDOCKER protocol. All bound waters and ligands were eliminated from the protein and the polar hydrogen was added to the proteins. Briefly, we define the EGFR complex as a receptor, then perform simulation of the compound into the ATP binding site in EGFR by replacing ATP molecule with our compound H20.
4. Conclusions
A series of benzohydrazide derivatives H1–H32 containing dihydropyrazoles were synthesized and evaluated in vitro for anticancer activities against the A549, MCF-7, HeLa and HepG2 cell lines, EGFR inhibitory activities, as well as MTT assays. These compounds exhibited potent EGFR inhibitory activities and antiproliferative activities against the above four cell lines. Among them, compound H20 showed the most potent EGFR inhibition activities (IC50 = 0.08 μM) and anticancer activities against A549, MCF-7, HeLa and HepG2 (IC50 = 0.46, 0.29, 0.15 and 0.21 μM, respectively). Docking simulation of the binding model of compound H20 with EGFR indicated that conventional hydrogen bonds and Van der Waals interactions with the protein residues in the ATP binding site might play a crucial role in its EGFR inhibition and antiproliferative activities. Therefore, compound H20 may be developed as a potential antitumor agent.
Acknowledgments
The work was financed by a grant (No. J1103512) from National Natural Science Foundation of China and the Projects (No. CXY1409 & CG1305) from the Science & Technology Bureau of Lianyungang City of Jiangsu Province.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/8/1012/s1.
Author Contributions
Haichao Wang and Hailiang Zhu conceived, designed and performed the synthetic experiment part; Hongxia Li analyzed the data; Xiaoqiang Yan conceived, designed and performed the docking simulation study part; Tianlong Yan conceived, designed and performed the pharmacological test part; Haichao Wang wrote the paper. Xiaoqiang Yan, Zhongchang Wang and Hailiang Zhu assisted with the paper revision.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds H1–H32 are available from the authors.
References
- 1.Farce A., Loge C., Gallet S., Lebegue N., Carato P., Chavatte P., Berthelot P., Lesieur D. Docking study of ligands into the colchicine binding site of tubulin. J. Enzyme Inhib. Med. Chem. 2004;19:541–547. doi: 10.1080/14756360412331280545. [DOI] [PubMed] [Google Scholar]
- 2.El-Nakkady S.S., Hanna M.M., Roaiah H.M., Ghannam I.A. Synthesis, molecular docking study and antitumor activity of novel 2-phenylindole derivatives. Eur. J. Med. Chem. 2012;47:387–398. doi: 10.1016/j.ejmech.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Citri A., Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa M.L., Lima L.M., Tesch R., Sant’Anna C.M., Totzke F., Kubbutat M.H., Schachtele C., Laufer S.A., Barreiro E.J. Novel 2-chloro-4-anilino-quinazoline derivatives as EGFR and VEGFR-2 dual inhibitors. Eur. J. Med. Chem. 2014;71:1–14. doi: 10.1016/j.ejmech.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Blume-Jensen P., Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 6.Cheng W., Zhu S., Ma X., Qiu N., Peng P., Sheng R., Hu Y. Design, synthesis and biological evaluation of 6-(nitroimidazole-1H-alkyloxyl)-4-anilinoquinazolines as efficient EGFR inhibitors exerting cytotoxic effects both under normoxia and hypoxia. Eur. J. Med. Chem. 2015;89:826–834. doi: 10.1016/j.ejmech.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Rego R.L., Foster N.R., Smyrk T.C., Le M., O’Connell M.J., Sargent D.J., Windschitl H., Sinicrope F.A. Prognostic effect of activated EGFR expression in human colon carcinomas: Comparison with EGFR status. Br. J. Cancer. 2010;102:165–172. doi: 10.1038/sj.bjc.6605473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao C., Lu S., Sowa A., Kivlin R., Amaral A., Chu W., Yang H., Di W., Wan Y. Priming with EGFR tyrosine kinase inhibitor and EGF sensitizes ovarian cancer cells to respond to chemotherapeutical drugs. Cancer Lett. 2008;266:249–262. doi: 10.1016/j.canlet.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsudomi T., Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277:301–308. doi: 10.1111/j.1742-4658.2009.07448.x. [DOI] [PubMed] [Google Scholar]
- 10.Park H.J., Lee K., Park S.J., Ahn B., Lee J.C., Cho H., Lee K.I. Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett. 2005;15:3307–3312. doi: 10.1016/j.bmcl.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 11.Park H.J., Lee K., Park S.J., Ahn B., Lee J.C., Cho H., Lee K.I. Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives. J. Med. Chem. 2004;47:3693–3696. doi: 10.1021/jm030394f. [DOI] [PubMed] [Google Scholar]
- 12.Sridhar R., Perumal P.T., Etti S., Shanmugam G., Ponnuswamy M.N., Prabavathy V.R., Mathivanan N. Design, synthesis and anti-microbial activity of 1H-pyrazole carboxylates. Bioorg. Med. Chem. Lett. 2004;14:6035–6040. doi: 10.1016/j.bmcl.2004.09.066. [DOI] [PubMed] [Google Scholar]
- 13.Bebernitz G.R., Argentieri G., Battle B., Brennan C., Balkan B., Burkey B.F., Eckhardt M., Gao J., Kapa P., Strohschein R.J., et al. The effect of 1,3-diaryl-[1H]-pyrazole-4-acetamides on glucose utilization in ob/ob mice. J. Med. Chem. 2001;44:2601–2611. doi: 10.1021/jm010032c. [DOI] [PubMed] [Google Scholar]
- 14.Li-Weber M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Neuhouser M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 16.Ducray R., Ballard P., Barlaam B.C., Hickinson M.D., Kettle J.G., Ogilvie D.J., Trigwell C.B. Novel 3-alkoxy-1H-pyrazolo[3,4-d]pyrimidines as EGFR and erbB2 receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2008;18:959–962. doi: 10.1016/j.bmcl.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Tao X.X., Duan Y.T., Chen L.W., Tang D.J., Yang M.R., Wang P.F., Xu C., Zhu H.L. Design, synthesis and biological evaluation of pyrazolyl-nitroimidazole derivatives as potential EGFR/HER-2 kinase inhibitors. Bioorg. Med. Chem. Lett. 2016;26:677–683. doi: 10.1016/j.bmcl.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Qiu K.M., Wang H.H., Wang L.M., Luo Y., Yang X.H., Wang X.M., Zhu H.L. Design, synthesis and biological evaluation of pyrazolyl-thiazolinone derivatives as potential EGFR and HER-2 kinase inhibitors. Bioorg. Med. Chem. 2012;20:2010–2018. doi: 10.1016/j.bmc.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 19.Lv P.C., Li H.Q., Sun J., Zhou Y., Zhu H.L. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agents. Bioorg. Med. Chem. 2010;18:4606–4614. doi: 10.1016/j.bmc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Lv P.C., Li D.D., Li Q.S., Lu X., Xiao Z.P., Zhu H.L. Synthesis, molecular docking and evaluation of thiazolyl-pyrazoline derivatives as EGFR TK inhibitors and potential anticancer agents. Bioorg. Med. Chem. Lett. 2011;21:5374–5377. doi: 10.1016/j.bmcl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J., Wang S., Luo Z., Qiu H., Wang P., Zhang X., Yang Y., Yin Y., Zhang F., Zhu H. Synthesis and biological evaluation of compounds which contain pyrazole, thiazole and naphthalene ring as antitumor agents. Bioorg. Med. Chem. Lett. 2014;24:2324–2328. doi: 10.1016/j.bmcl.2014.03.072. [DOI] [PubMed] [Google Scholar]
- 22.Rasolofonjatovo E., Provot O., Hamze A., Rodrigo J., Bignon J., Wdzieczak-Bakala J., Desravines D., Dubois J., Brion J.D., Alami M. Conformationnally restricted naphthalene derivatives type isocombretastatin A-4 and isoerianin analogues: Synthesis, cytotoxicity and antitubulin activity. Eur. J. Med. Chem. 2012;52:22–32. doi: 10.1016/j.ejmech.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Rajabi M., Khalilzadeh M.A., Tavakolinia F., Signorelli P., Ghidoni R., Santaniello E. Naphthalene-fused (alpha-alkoxycarbonyl)methylene-gamma-butyrolactones: Antiproliferative activity and binding to bovine serum albumin and DNA. DNA Cell Biol. 2012;31:783–789. doi: 10.1089/dna.2011.1433. [DOI] [PubMed] [Google Scholar]
- 24.Muhlbacher J.M. Naftifine: A topical allylamine antifungal agent. Clin. Dermatol. 1991;9:479–485. doi: 10.1016/0738-081X(91)90076-W. [DOI] [PubMed] [Google Scholar]
- 25.Altintop M.D., Atli O., Ilgin S., Demirel R., Ozdemir A., Kaplancikli Z.A. Synthesis and biological evaluation of new naphthalene substituted thiosemicarbazone derivatives as potent antifungal and anticancer agents. Eur. J. Med. Chem. 2016;108:406–414. doi: 10.1016/j.ejmech.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 26.Shear N.H., Villars V.V., Marsolais C. Terbinafine: An oral and topical antifungal agent. Clin. Dermatol. 1991;9:487–495. doi: 10.1016/0738-081X(91)90077-X. [DOI] [PubMed] [Google Scholar]
- 27.Karakurt A., Alagoz M.A., Sayoglu B., Calis U., Dalkara S. Synthesis of some novel 1-(2-naphthyl)-2-(imidazol-1-yl)ethanone oxime ester derivatives and evaluation of their anticonvulsant activity. Eur. J. Med. Chem. 2012;57:275–282. doi: 10.1016/j.ejmech.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Tzeng C.C., Lee K.H., Wang T.C., Han C.H., Chen Y.L. Synthesis and cytotoxic evaluation of a series of gamma-substituted gamma-aryloxymethyl-alpha-methylene-gamma-butyrolactones against cancer cells. Pharm. Res. 2000;17:715–719. doi: 10.1023/A:1007534416561. [DOI] [PubMed] [Google Scholar]
- 29.Zeglis B.M., Divilov V., Lewis J.S., Divilov J.S. Lewis, Role of metalation in the topoisomerase IIalpha inhibition and antiproliferation activity of a series of alpha-heterocyclic-N4-substituted thiosemicarbazones and their Cu(II) complexes. J. Med. Chem. 2011;54:2391–2398. doi: 10.1021/jm101532u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder A.K., Adhikary N., Maity M.K., Jha T. Synthesis, pharmacological activity and comparative QSAR modeling of 1,5-N,N′-substituted-2-(substituted naphthalenesulphonyl) glutamamides as possible anticancer agents. Eur. J. Med. Chem. 2010;45:1760–1771. doi: 10.1016/j.ejmech.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Lokhande T.N., Viswanathan C.L., Joshi A., Juvekar A. Design, synthesis and evaluation of naphthalene-2-carboxamides as reversal agents in MDR cancer. Bioorg. Med. Chem. 2006;14:6022–6026. doi: 10.1016/j.bmc.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Reddy G.R., Kuo C.C., Tan U.K., Coumar M.S., Chang C.Y., Chiang Y.K., Lai M.J., Yeh J.Y., Wu S.Y., Chang J.Y., et al. Synthesis and structure-activity relationships of 2-amino-1-aroylnaphthalene and 2-hydroxy-1-aroylnaphthalenes as potent antitubulin agents. J. Med. Chem. 2008;51:8163–8167. doi: 10.1021/jm8008635. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H.L., Yan X.Q, Wang Q.Z, Wang Z.C. The Preparation Methods of a Series of Novel Dihydrogen Pyrazole Morpholine Derivatives Containing Naphthalene Ring Skeleton. CN201510268943.7. Chinese Patent. 2015 Oct 26;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.