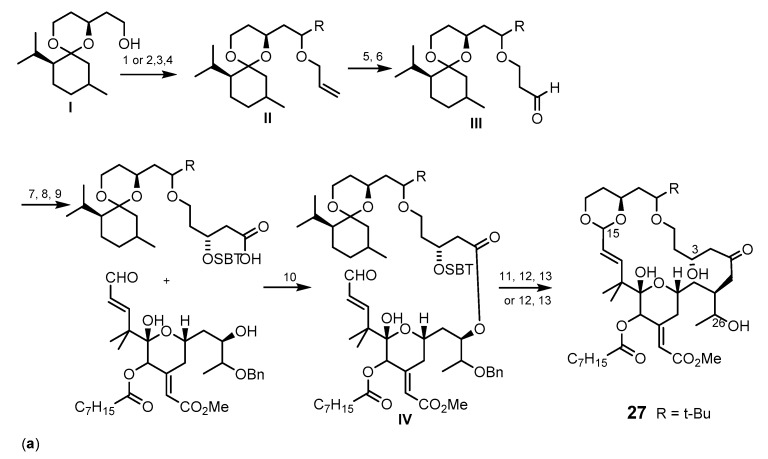
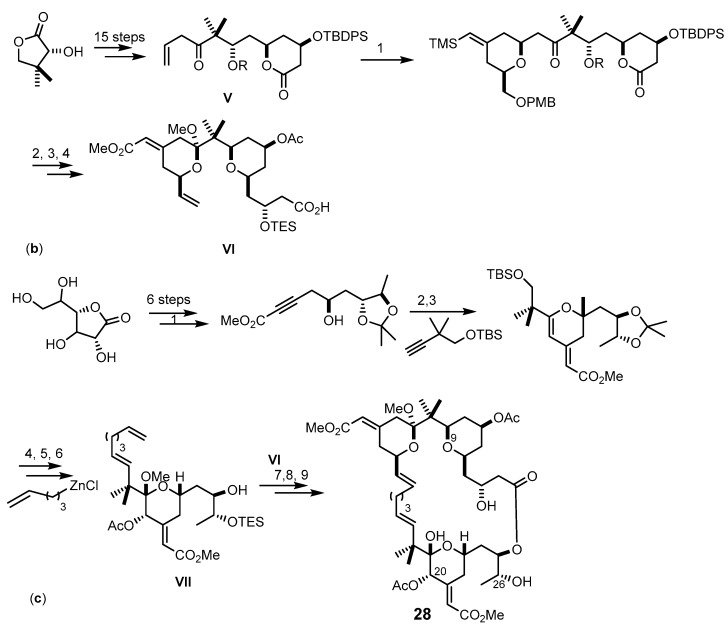
Scheme 4.
Synthesis of the methyl bryostatin 1 (25) analogs 27 (a) and 28 (b,c). Reagents and conditions: (a) (1) NaH, allybromide, THF, rt; (2) Dess-Martin periodinane, CH2Cl2, rt; (3) t-BuLi, Et2O, −78 °C, (recyclable 1:1 diastereomer mixture: Dess-Martin periodinane, CH2Cl2, rt, then NaBH4, CeCl3·7H2O, −40 °C; (4) t-BuOH, allyl bromide, THF, rt; (5) 9-BBN, THF, 66 °C, then NaOH, H2O2; (6) Dess-Martin periodinane, CH2Cl2, rt; (7) (−)-(lpc)BOMe, allylmagnesium bromide, CH2Cl2, −78 °C to rt; (8) TBSCl, imidazole, THF, rt; (9) catalytic KMnO4, NaIO4, rt; (10) 2,4,6-trichloro-benzoylchloride, Et3N, DMAP, CH2Cl2, rt; (11) HF·pyridine, CH3CN, rt; (12) Amberlyst-15 resin, CH2Cl2, rt; (13) Pd(OH)2, H2, EtOAc, 1 atm; (b) (1) 10 mol % of [CpRu(CH3CN)3]PF6, acetone, rt; (2) NBS, DMF then BF3-OEt2, 1,3-propanedithiol, CH2Cl2, 0 °C then PPTS, CH3OH, CH(OCH3)3, refux; (3) TESCl, DMAP, then pyridine, Ac2O; PPTS, MeOH, rt; DMSO, (COCl)2, Et3N, CH2Cl2 −78 °C; Ph3PCH3Br, n-BuLi; (4) TBAF, THF, rt; Me3SnOH, DCE, 140 °C, microwave; Pd(PPh3)4, CO, DMF/CH3OH, 85 °C; TESOTf, 2,6-lutidine, CH2Cl2; (c) (1) n-BuLi, methyl propionate, BF3·OEt2, THF, −78 °C; (2) Pd(OAc)2, tris(2,6-dimethoxyphenyl) phosphine, benzene, then Pd(O2CCF3)2, rt; (3) trifluoroperacetic acid, NaHPO4, CH2Cl2/CH3CN/ CH3OH, 0 °C; Dess-Martin oxidation; NaBH4, CeCl3·7H2O, −30o°C; Ac2O, pyridine, DMAP, CH2Cl2, rt; (4) CrCl2, CHI3, THF, rt; 26% (47% BRSM); Pd(PPh3)4, THF, rt; (5) AcOH/H2O; (6) TESCl, ETA, DMF, −35 to −15 °C; (7) Et3N, DMAP, 2-mehyl-6-nitrobenzoic acid anhydride, CH2Cl2; (8) benzene, 50–80 °C, 17 mol % of Grubbs-Hoveyda catalyst; (9) PPTS, MeOH, rt.