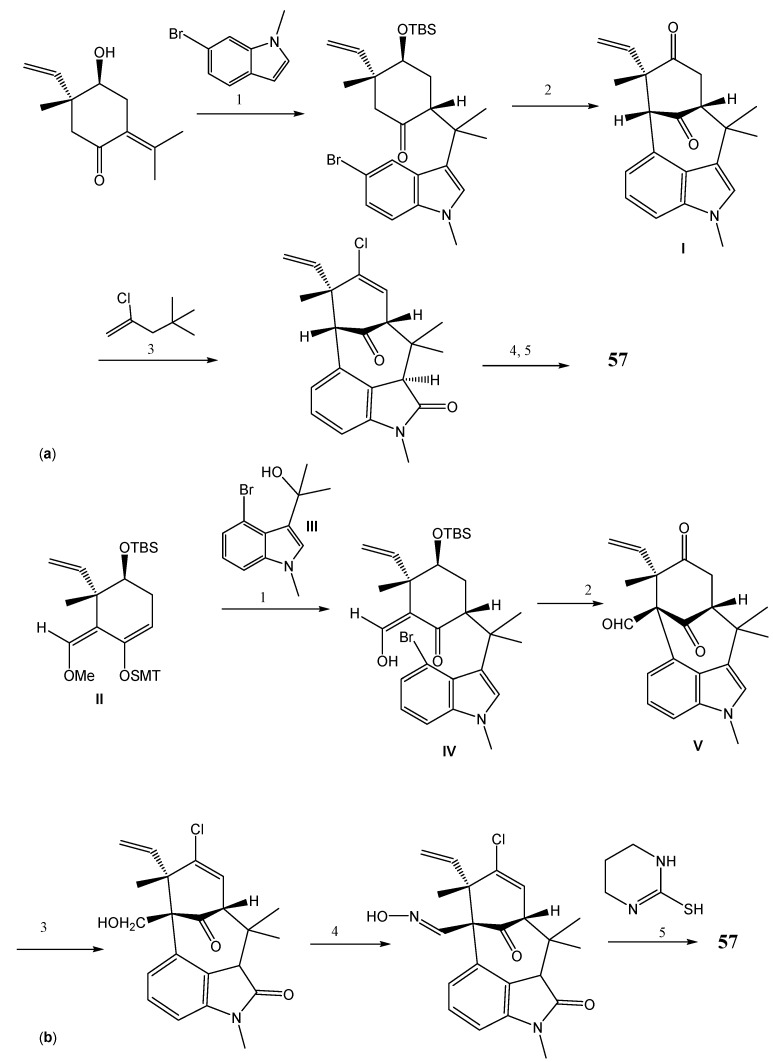
Scheme 11.
Synthesis of N-methylwelwitindolinone C isothiocyanate (57) by Gang’s approach (a) and Rawal’s approach (b). Reagents and conditions: (a) (1) iodine promoted bromination; (2) NaNH2, t-BuOH, THF; (3) trimethyethylstannane; (4) LiEt3B-D, THF, Cl3CCONCO, CH2Cl2, K2CO3, MeOH; AgOTf, PhI(OAc)2, CH3CN, bathophenantroline; (5) NaH, air, THF; (b) (1) TiCl4, toluene; (2) Pd(OAc)2, P-tBu3, KO-tBu, toluene; (3) NaBH(OMe)3, THF/EtOH then N2H2, AcOH, EtOH; NCS, pyridine; MMPP, TFA, AcOH; (4) Dess-Martin periodinane, NaHCO3, CH2Cl2; NH2OH.HCl, pyridine, MeOH; (5) NCS, DMF, THF, then Et3N.