Table 1.
Structure of native CDs and common modified CDs.
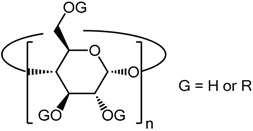 | |||
|---|---|---|---|
| Abbreviation | n | Substituent (R) | Number of R Group by CD |
| α-CD | 6 | (−) | 0 |
| β-CD | 7 | (−) | 0 |
| γ-CD | 8 | (−) | 0 |
| HPαCD | 6 | -CH2-CHOH-CH3 | 3.6 |
| RAMEα | 6 | -CH3 | 10.8 |
| HPβCD | 7 | -CH2-CHOH-CH3 | 5.6 |
| KLEPTOSE® CRYSMEB | 7 | -CH3 | 4 |
| Methyl-β-CD | 7 | -CH3 | 1.6 |
| RAMEβ | 7 | -CH3 | 12.6 |
| SBE7-β-CD | 7 | -(CH2)4-SO3Na | 7 |
| TRIMETHYL-β-CD | 7 | -CH3 | 21 |
| HPγCD | 8 | -CH2-CHOH-CH3 | 4.8 |
| RAMEγ | 8 | -CH3 | 14.4 |
α-CD, α-cyclodextrin; β-CD, β-cyclodextrin; CD, cyclodextrin; CRYSMEB, crystalline methylated-β-cyclodextrin; γ-CD, γ-cyclodextrin; HPαCD, 2-hydroxypropyl-α-cyclodextrin; HPβCD, 2-hydroxypropyl-β-cyclodextrin; HPγCD, 2-hydroxypropyl-γ-cyclodextrin; Methyl-β-CD, methyl-β-cyclodextrin ; RAMEα, randomly-methylated-α-cyclodextrin; RAMEβ, randomly-methylated-β-cyclodextrin; RAMEγ, randomly-methylated-γ-cyclodextrin; SBE7-β-CD, sulfobutylether-7-β-cyclodextrin; TRIMETHYL-β-CD, TRIMETHYL-β-cyclodextrin.
