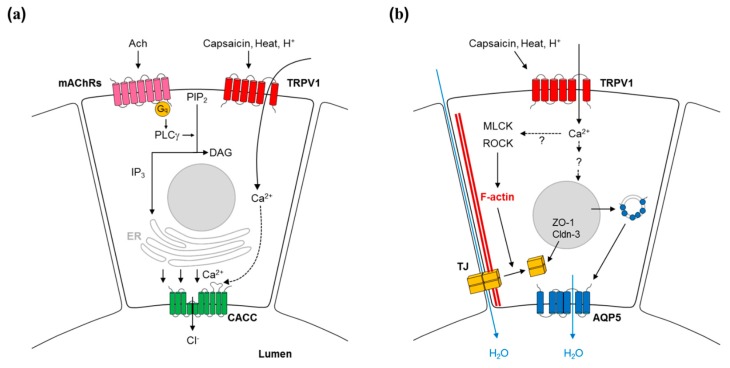
Figure 1.
Capsaicin mediates saliva secretion via transient receptor potential vanilloid subtype 1 (TRPV1). (a) Calcium-activated chloride channel (CACC)-induced saliva secretion. Activation of Muscarinic acetylcholine receptors (mAChRs) via acetylcholine (Ach) mediates G-protein coupled signaling downstream, including the phospholipase Cg (PLCg) pathway. The generated inositol 1,4,5-triphosphate (IP3) by PLCg induces Ca2+ mobilization from ER storage. Capsaicin increased [Ca2+]i level by increasing the cation conductance of TRPV1. The increased [Ca2+]i promotes the saliva secretory process by activating CACC, leading the efflux of chloride ions toward ductal space, and the subsequent generation of an electrochemical gradient beyond the apical border of acinar cells. (b) The role of TRPV1 in trans- and paraceulluar routes. TRPV1 activation enhances paracellular permeability by regulating the spatial distribution of F-actin linked to tight junction (TJ). The expression level of TJ proteins—including zonula occludin (ZO)-1 and Claudin (Cldn)-3—is also increased by TRPV1 activation. In the transcellular route, capsaicin treatment enhances the aquaporin 5 (AQP5) trafficking to the plasma membrane and its mRNA expression level. However, the accurate mechanism underlying AQP5 trafficking is still unclear. Broken lines are used for tentative interactions. For details, see text. PIP2: Phosphatidylinositol 4,5-bisphosphate; DAG: diacylglycerol; MLCK: myosin light chain kinase; ROCK: RhoA-Rho-associated protein kinase.