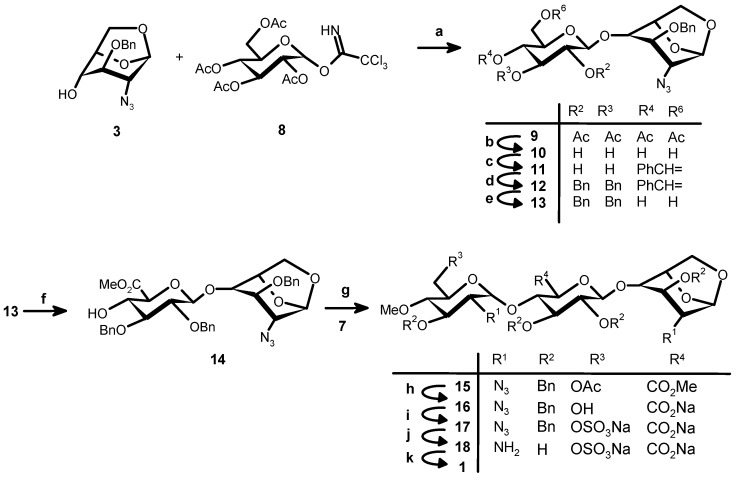
Scheme 2.
Synthesis of 1. Reagents and conditions: (a) BF3:Et2O, 4 Å MS, CH2Cl2, −30 °C, 2 h, 77%; (b) 0.5 M MeONa, THF-MeOH, RT, 1 h; (c) PhCH(OMe)2, CSA, DMF, RT, 17 h, 56% (2 steps); (d) BnBr, NaH, DMF, RT, 2 h; (e) 70% aq. TFA, CH2Cl2, RT, 4 h; (f) (i) Dibromantin, TEMPO, CH3CN, RT, 2.5 h; (ii) CH3I, NaHCO3, DMF, RT, 16 h, 82% (4 steps); (g) TMSOTf, 4 Å MS, CH2Cl2, −20 °C, ~2 h, 58% (+ β-anomer 20%); (h) LiOH, H2O2, THF-MeOH, 16 h, 82%; (i) SO3:C5H5N, C5H5N, 55 °C, 2 h, 76%; (j) H2, 10% Pd/C, tBuOH-H2O, 60 h, 100%; (k) SO3:C5H5N, NaOH, RT, 16 h, 57%.