Abstract
Trillium govanianum rhizome is used as an analgesic and anti-inflammatory remedy in traditional medicine in northern Pakistan. In an attempt to establish its medicinal value, the present research evaluated the analgesic and anti-inflammatory potential of T. govanianum. The in vivo anti-inflammatory activity of extract and fractions was investigated in the carrageenan induced paw edema assay. The in vitro suppression of oxidative burst of extract, fractions and isolated compounds was assessed through luminol-enhanced chemiluminescence assay. The in vivo analgesic activity was assayed in chemical and thermal induced nociceptive pain models. The crude methanol extract and its solvent fractions showed anti-inflammatory and analgesic responses, exhibited by significant amelioration of paw edema and relieve of the tonic visceral chemical and acute phasic thermal nociception. In the oxidative burst assay, based on IC50, the crude methanol extract and n-butanol soluble fraction produced a significant inhibition, followed by chloroform and hexane soluble fractions as compared to ibuprofen. Similarly, the isolated compounds pennogenin and borassoside E exhibited significant level of oxidative burst suppressive activity. The in vivo anti-inflammatory and analgesic activities as well as the in vitro inhibition of oxidative burst validated the traditional use of T. govanianum rhizomes as a phytotherapeutic remedy for both inflammatory conditions and pain. The observed activities might be attributed to the presence of steroids and steroid-based compounds. Therefore, the rhizomes of this plant species could serve as potential novel source of compounds effective for alleviating pain and inflammation.
Keywords: Trilliaceae, anti-inflammatory, analgesic, oxidative burst, pennogenin, borassoside E
1. Introduction
It is highly desired to discover excellent remedies for diseases that are economical, having no or low side effects, potent and efficacious in various pathological conditions. For discovering such products, medicinal plants and herbal medicines can be the best choice as plants produce a wide range of bioactive compounds, making them a rich source of different types of medicines [1]. Numerous drugs have entered international pharmacopoeias through ethnopharmacological and traditional medicine studies [2]. Based on folkloric use, research on medicinal plants has proved the presence of valuable pharmacologically active compounds with anti-cancer, antiparasitic, antifungal, antibacterial, analgesics, and anti-inflammatory properties [3,4,5]. In developing countries, majority of population depends largely on traditional system of medicines because of their low cost and ease of availability [6].
At present, available drugs for the management of pain and inflammatory conditions are either narcotic, non-narcotic analgesics or corticosteroids, possessing well known side and adverse effects [7]. Furthermore, synthetic drugs are very expensive to develop since, for the successful introduction of a new drug product hundreds and thousands of compounds are to be synthesized, screened and tested which involves a huge expense as well as time. On the other side many medicines of plant origin have been used since long with minimum or none side and adverse effects [8]. It is, therefore, inevitable to search medicinal plants for effective analgesic and anti-inflammatory molecules with significant therapeutic potentials.
Trillium govanianum belongs to the genus Trillium (family: Melanthiaceae alt. Trilliaceae) is an indigenous medicinal plant of Pakistan, found at an altitudinal range of 2500–3800 m [9]. In traditional medicines, rhizomes of this plant species are used for treating wounds, dysentery, skin boils, infections, and menstrual and sexual disorders [10,11,12]. The genus Trillium consists of 31 species, widely distributed from the western Himalayas to Japan, China, Kamchatka (Russia) and North America [13] and is an important source of bioactive compounds of different classes like steroids, glycosides, terpenoids, sterols, saponins, sapogenins and flavonoids [14,15,16]. Literature studies indicate that plant species of this genus have been extensively used as remedy for various diseases like T. tschonoskii has been traditionally used in China, for at least one thousand years, for treatment of neurasthenia, giddiness, headache, removing carbuncles and ameliorating pains [17,18,19]. The marked inhibitory activity against COX-2 production in macrophagocytes of the mouse abdominal cavity by isolated compounds from T. tschonoskii has also been reported [20]. It has also been described that the ethanol, ethyl acetate and n-butanol extracts of T. tschonoskii significantly suppress carrageenan-induced edema in rats [21]. The rhizomes of T. erectum called ‘beth root’ have been used in folk medicine for the treatment of hemorrhages of uterus, urinary tract and lungs [22].
Phytochemical analyses of T. govanianum rhizomes have resulted in isolation of steroids and saponins from the chloroform and n-butanol soluble fractions [23]. Moreover, antifungal and anticancer activities in addition to ethnomedicinal relevance of T. govanianum have also been recently reported [24,25]. However, to best of our knowledge studies concerning the therapeutic efficacy of T. govanianum as an analgesic and anti-inflammatory agent have not been carried out. Therefore, in present study, we evaluated the crude methanol extract and solvent soluble fractions thereof for analgesic and anti-inflammatory activities, using in vivo animal models, while the isolated compounds in our previous study [23] were tested for inhibition of oxidative burst using luminol-enhanced chemiluminescence assay.
2. Results and Discussion
2.1. Acute Toxicity
2.1.1. In Vivo Toxicity Test
No overt behavioral change was reported during an observation time of 2 h post crude methanol extract administration. As shown in Table 1, maximum mortality was observed at a dose of 6000 mg/kg while no lethality was observed at 500 mg/kg dose. From the LD50 value (2030.4 mg/kg), the crude methanolic extract is considered as safe to the maximum of tested dose used in this study.
Table 1.
In vivo toxicity test of Trillium govanianum rhizome methanol extract.
| Dose (mg/kg) | Total Number of Mice = 6 | Percent Lethality | LD50 (mg/kg) | |
|---|---|---|---|---|
| No. of Dead Mice | No. of Survived Mice | |||
| 150 | 0 | 6 | 0 | 2030.4 (1488.8–3069.0) * |
| 500 | 0 | 6 | 0 | |
| 1000 | 1 | 5 | 16 | |
| 1500 | 2 | 4 | 33 | |
| 3000 | 5 | 1 | 83 | |
| 6000 | 6 | 0 | 100 | |
* 95% confidence limit in parentheses.
2.1.2. In Vitro Toxicity Test
The crude methanolic extract and its fractions were tested in vitro against NIH 3T3 mouse embryo fibroblast cell line to ascertain their cytotoxicity. As shown in Table 2, the IC50 values for the in vitro assay were in following order: cycloheximide < BuOH-fr < Aq-fr < Chl-fr < EtOAc fraction < Hex-fr < MeOH-ext. The cytotoxicity decreased in the following rank order: MeOH-ext > Hex-fr > EtOAc-fr > Chl-fr > Aq-fr > BuOH-fr > cycloheximide. The standard cycloheximide was more cytotoxic to fibroblasts compared to crude extract and its fractions.
Table 2.
In vitro cytotoxicity assay of Trillium govanianum rhizome methanol extract and its fractions on NIH 3T3 mouse embryo fibroblasts.
| Sample | IC50 (µg/mL) |
|---|---|
| MeOH-ext | 7.89 ± 0.43 |
| Hex-fr | 5.78 ± 0.34 |
| Chl-fr | 3.69 ± 0.77 |
| EtOAc-fr | 3.95 ± 0.25 |
| BuOH-fr | 2.59 ± 0.14 |
| Aq-fr | 3.07 ± 0.29 |
| Cycloheximide | 0.73 ± 0.12 |
Values are expressed as mean ± SD of three separate experiments.
2.2. Anti-Inflammatory Activity
The anti-inflammatory response of T. govanianum rhizomes methanol extract (MeOH-ext) and fractions thereof in carrageenan-induced paw edema model is presented in Table 3 The results indicate that MeOH-ext and its fractions at 50, 100 and 200 mg/kg exhibit significant anti-inflammatory activity comparable to the standard anti-inflammatory drug, diclofenac sodium.
Table 3.
Anti-inflammatory activity of Trillium govanianum rhizome methanol extract and fractions in carrageenan-induced paw edema assay.
| Sample | Dose (mg/kg) | 1st h | 2nd h | 3rd h | 4th h | 5th h |
|---|---|---|---|---|---|---|
| Diclofenac sodium | 10 | 27.3 ± 2.7 *** | 47.6 ± 0.8 *** | 67.0 ± 2.5 *** | 70.6 ± 0.6 *** | 74.3 ± 0.6 *** |
| MeOH-ext | 50 | 8.0 ± 1.7 * | 18.0 ± 1.7 ** | 22.6 ± 2.3 *** | 32.0 ± 5.2 *** | 34.0 ± 5.2 *** |
| 100 | 12.0 ± 1.1 ** | 44.3 ± 4.6 *** | 65.0 ± 4.6 *** | 63.6 ± 1.7 *** | 66.3 + 4.6 *** | |
| 200 | 19.0 ± 2.3 * | 44.6 ± 3.8 *** | 62.6 ± 3.7 *** | 62.6 ± 1.4 *** | 64.6 ± 4.0 *** | |
| Chl-fr | 25 | 4.0 ± 1.0 * | 8.5 ± 0.5 * | 21.3 ± 3.5 ** | 35.0 ± 1.0 *** | 42.5 ± 2.5 *** |
| 50 | 4.0 ± 2.0 ** | 18.8 ± 1.0 ** | 20.3 ± 2.0 ** | 43.0 ± 1.0 *** | 58.0 ± 1.0 *** | |
| 100 | 12.1 ± 3.0 * | 21.5 ± 0.5 ** | 43.5 ± 3.5 *** | 45.0 ± 1.0 *** | 63.5 ± 0.5 *** | |
| EtOAc-fr | 25 | 3.5 ± 0.5 * | 9.5 ± 0.5 * | 18.0 ± 3.0 ** | 18.5 ± 1.5 ** | 39.5 ± 0.5 *** |
| 50 | 10.5 ± 0.5 * | 16.0 ± 1.0 ** | 29.5 ± 0.5 *** | 30.5 ± 2.5 *** | 44.5 ± 3.5 *** | |
| 100 | 9.5 ± 0.5 * | 22.5 ± 1.5 ** | 30.5 ± 0.5 *** | 33.0 ± 2.0 *** | 47.5 ± 0.5 *** | |
| BuOH-fr | 25 | 12.9 ± 3.7 * | 14.3 ± 5.5 * | 35.2 ± 1.0 *** | 58.0 ± 1.5 *** | 61.0 ± 3.4 *** |
| 50 | 18.8 ± 3.4 *** | 46.6 ± 4.9 *** | 68.6 ± 2.3 *** | 66.0 ± 1.7 *** | 69.0 ± 4.3 *** | |
| 100 | 25.6 ± 1.7 *** | 47.6 ± 2.1 *** | 68.6 ± 1.4 *** | 70.3 ± 2.6 *** | 72.6 ± 3.9 *** |
Percent inhibition of paw edema is expressed as mean ± SEM. One-way ANOVA followed by Dunnett’s post hoc test: * p < 0.05, ** p < 0.0, *** p < 0.001 compared to saline control; N = 6 mice per group.
The MeOH-ext and its fractions at doses of 100 and 200 mg/kg showed an anti-inflammatory activity that became significant (p < 0.01) in the second phase of inflammation, i.e., 2 h after the injection of carrageenan. The anti-inflammatory effect remained significant in the second phase with a maximum percent inhibitions of 64.6 ± 4.0, 63.5 ± 0.5, 47.5 ± 0.5 and 72.6 ± 3.9 by MeOH-ext, chloroform, ethyl acetate and n-butanol fractions respectively. The MeOH-ext and its fractions showed a slightly weaker activity in early phase of inflammation (1–2 h), though n-butanol fraction was active at a dose of 100 mg/kg.
In chemiluminescence assay, the in vitro inhibitory effects on the release of reactive oxygen species (ROS) from whole blood by MeOH-ext and its fractions are presented in Table 4. The results show that n-butanol fraction exhibit significant inhibition of oxidative burst for whole blood followed by MeOH-ext with IC50 ± SD of 16.5 ± 7.5 and 30.8 ± 7.0 µg/mL respectively.
Table 4.
Inhibitory effect on release of ROS by Trillium govanianum methanol extract, fractions and isolated compounds.
| Samples | IC50 ± SD (µg/mL) |
|---|---|
| MeOH-ext | 30.8 ± 7.0 |
| Hex-fr | 107.1 ± 38.4 |
| Chl-fr | 81.6 ± 24.6 |
| EtOAc-fr | 114.8 ± 2.3 |
| BuOH-fr | 16.5 ± 7.5 |
| Pennogenin | 5.0 ± 0.8 |
| Borassoside E | 31.5 ± 6.6 |
| Diosgenin | 53.2 ± 2.7 |
| Ibuprofen (Positive control) | 11.2 ± 1.9 |
Values represent mean ± SEM of three independent experiments.
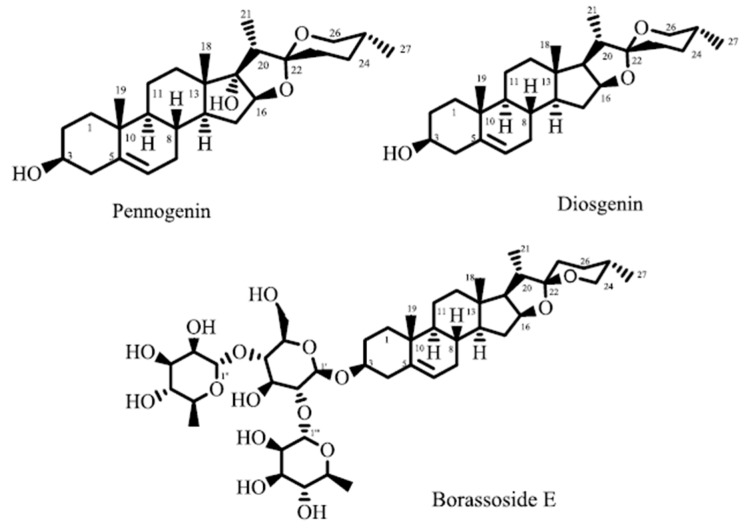
Based on these results, isolated compounds i.e., diosgenin, pennogenin and borassoside E (Figure 1) from T. govanianum rhizomes were screened for inhibitory effects on release of ROS from whole blood (Table 4). Pennogenin exhibited significant in vitro inhibitory effect by controlling release of ROS with IC50 of 5.0 ± 0.8 µg/mL, while borassoside E and diosgenin showed weak inhibition.
Figure 1.
Compounds of T. govanianum rhizomes exhibiting oxidative burst inhibitory effect.
Inflammation and reactive oxygen species (ROS) has a relationship of mutual promotion. ROS are associated with inflammatory response and may contribute tissue damages [26]. In addition, oxidative stress is thought to play an important role in the pathogenesis of many chronic inflammatory diseases by different molecular mechanisms [27]. Numerous studies have shown that production of reactive species such as ROS, reactive nitrogen species (RNS) and hypochlorous acid (HOCl) occur at the site of inflammation, thus exacerbating tissue damage as well as the inflammatory cascade [28,29].
Drugs that inhibit the formation or release of these toxic reactive species (or detoxify them) are effective in treating a variety of diseases that involves stimulation of immune cells like AIDS, rheumatoid arthritis and cancer [30]. Luminol-enhanced chemiluminescence assay is based on the detection of intracellular ROS released by opsonized zymosan activated immune cells. A measurement of chemiluminescence is an efficient and highly sensitive method to investigate different kinds of ROS, and is also a suitable method for detection of superoxide anion in biological systems [31].
The carrageenan-induced paw edema model, represent a form of acute inflammation, has been frequently used to assess the anti-inflammatory effects of natural products [32].This is useful model to assess the contribution of mediators involved in vascular changes associated with acute inflammation [33]. Within first hour following carrageenan injection, edema is induced by the release of various mediators such as histamine, bradykinin and 5-HT (5-hydroxytriptamine or serotonin), but not through prostaglandins (PG). These mediators, following activation of their receptors on endothelial cells, trigger iNOS (inducible nitric oxide synthase) activation and the generation of nitric oxide (NO), and RNS. In mice, following the intraplantar injection of carrageenan, tissue necrosis factor as well as cytokines such as IL-1 and IL-2 are produced [34]. Cyclooxygenase-2 (COX-2) is also induced within 2 h post carrageenan administration [35]. The NOS and COX pathways appear to operate together to amplify the inflammatory response. The dual inhibition of NO and PG obtained with NOS inhibitors could account for their marked anti-inflammatory activity [33]. Neutrophil infiltration in response to carrageenan and following membrane NADPH oxidase activation generates an oxygen respiratory burst giving rise to oxygen-derived free radicals and eliciting immune cell recruitment, which, in turn, produces tissue damage [36].
The ability of T. govanianum rhizomes MeOH-ext, fractions and isolated compounds thereof to inhibit release of ROS from whole blood after serum opsonized zymosan activation, may be a consequence of either scavenging of ROS during oxidative burst or inhibition of enzymes involved in their production such as NADPH oxidase, superoxide dismutase (SOD), catalases and peroxidases. As there is correlation between in vitro anti oxidative activities and in vivo anti-inflammatory activities [37], thus our in vitro results strongly support significant in vivo anti-inflammatory activity by extract and fractions in the carrageenan-induced paw edema model. Furthermore, diosgenin, a saponin aglycon found in a variety of plants, has anti-inflammatory properties, and it has been reported that diosgenin at dose of 400 µg/kg is responsible for maximum anti-inflammatory effect (82.25%) in carrageenan induced paw edema model [38]. Since, diosgenin is one of the major metabolite of T. govanianum rhizomes [23], it confirms our in vitro and in vivo anti-inflammatory results.
2.3. Analgesic Activity
2.3.1. Abdominal Constriction Assay (Tonic Visceral Chemically-Induced Nociception)
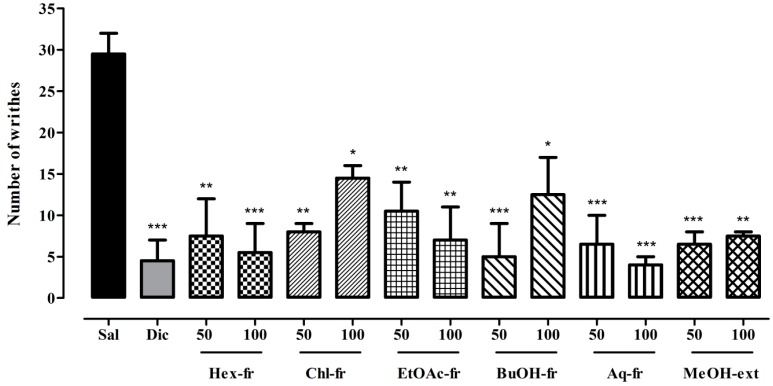
The results of MeOH-ext and its solvent soluble fractions in tonic visceral chemical-induced nociception assay are presented in Figure 2 (Table S1; Supplementary materials), which indicates that 50 and 100 mg/kg doses significantly attenuate acetic acid induced writhes for Hex-fr (p < 0.01, p < 0.001), Chl-fr (p < 0.01, p < 0.05), EtOAc-fr (p < 0.01), BuOH-fr (p < 0.001, p < 0.05), Aq-fr (p < 0.001), and MeOH-ext (p < 0.001, p < 0.01), respectively. The antinociceptive activity was comparable to the standard drug diclofenac sodium, which significantly relieved (p < 0.001) the tonic visceral chemical-induced nociceptive pain.
Figure 2.
Antinociceptive activity of Trillium govanianum rhizome methanol extract and fractions in tonic visceral chemically-induced nociception. Values are expressed as mean ± SEM. ANOVA followed by Dunnett’s post hoc test: * p < 0.05, ** p < 0.01, *** p < 0.001 compared to saline treated group; N = 6. Dic = diclofenac.
Abdominal constriction assay (acetic acid-induced writhing method) is widely used for evaluation of peripheral anti-nociceptive activity [39]. In tonic visceral chemical-induced nociception model, injection of acetic acid into the peritoneal cavity of mice has been attributed to the release of arachidonic acid (AA), which contributes in prostaglandin synthesis through cyclooxygenase enzyme [8]. The special nerve endings that sense pain are very sensitive to prostaglandin. When prostaglandins are released, nerve endings respond to it through prostaglandin E2 receptor, transmitting the pain and injury messages to brain and induce strong contraction tracked by extension of the hind limbs (writhing). This visceral pain model is simple, reliable and rapid for investigation of peripheral analgesics.
2.3.2. Hot Plate Test (Acute Phasic Thermal Nociception)
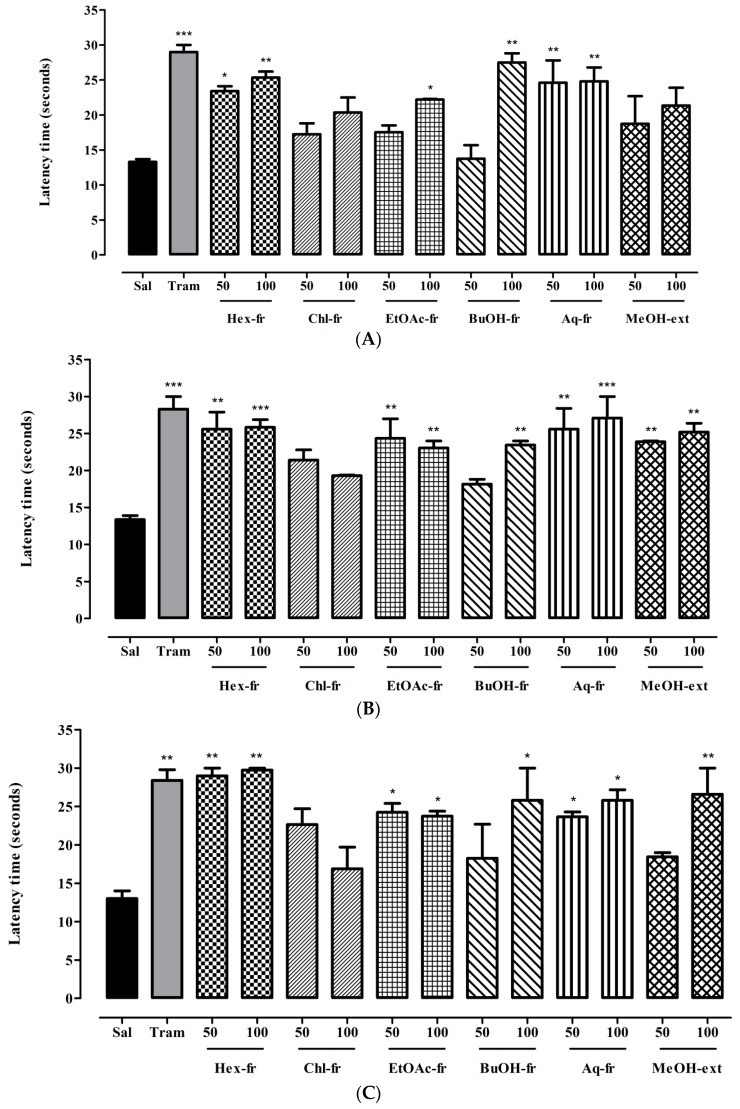
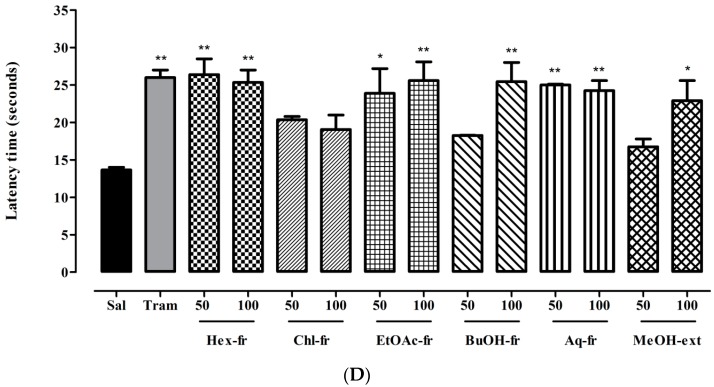
The MeOH-ext and its fractions were evaluated for analgesic effect in the acute phasic thermal nociceptive pain. The results shown in Figure 3A–D (Table S2; Supplementary materials) indicate that, after 30 min, compared to normal saline, significant attenuation of thermal-induced nociception was observed for Hex-fr at 50 mg/kg (p < 0.05) and 100 mg/kg (p < 0.01), EtOAc-fr at 100 mg/kg (p < 0.05), BuOH-fr at 100 mg/kg (p < 0.01), and Aq-fr at 50 and 100 mg/kg (p < 0.01) (Figure 3A). After 60 min, significant analgesic effect was observed for Hex-fr at 50 mg/kg (p < 0.01) and 100 mg/kg (p < 0.001), EtOAc-fr at 50 and 100 mg/kg (p < 0.01), BuOH-fr at 100 mg/kg (p < 0.01), Aq-fr at 50 mg/kg (p < 0.01) and 100 mg/kg (p < 0.001), and MeOH-ext at both doses (p < 0.01) (Figure 3B). Likewise, significant protection against thermal-induced nociception after 90 min was observed with all test doses of Hex-fr (p < 0.01), EtOAc-fr (p < 0.05), Aq-fr (p < 0.05) as well as with 100 mg/kg dose of BuOH-fr (p < 0.05) and MeOH-ext (p < 0.01) (Figure 3C). Moreover, analgesic effect produced after 120 min was significant for all test doses of Hex-fr (p < 0.01), EtOAc-fr (p < 0.05, p < 0.01) and Aq-fr (p < 0.01), and for only 100 mg/kg doses of BuOH-fr (p < 0.01) and MeOH-ext (p < 0.05) (Figure 3D).
Figure 3.
(A) Antinociceptive effect of Trillium govanianum rhizomes MeOH-extract its fractions (mg/kg) after 30 min. Sal, saline solution; Tram, tramadol; (B) Antinociceptive effect of Trillium govanianum rhizomes MeOH-extract its fractions (mg/kg) after 60 min. Sal, saline solution; Tram, tramadol; (C) Antinociceptive effect of Trillium govanianum rhizomes MeOH-extract its fractions (mg/kg) after 90 min. Sal, saline solution; Tram, tramadol; (D) Antinociceptive effect of Trillium govanianum rhizomes MeOH-extract its fractions (mg/kg) after 120 min. Sal, saline solution; Tram, tramadol. ANOVA followed by Dunnett’s post hoc test: * p < 0.05, ** p < 0.01, *** p < 0.001 compared to saline treated group; N = 6.
Hot plate test is one of the most common tests employed in evaluation of central analgesic potential of drugs. The mice paws are very sensitive to heat at temperatures, which are not damaging to skin. Mice show escape responses to the nocifensive stimulus and are manifested as jumping, licking or withdrawal of the paws. These responses take prolonged time to appear after administration of centrally acting analgesic drugs. Thus, the hot plate test model measures different responses to acute nociceptive or non-inflammatory inputs and is one of the models normally used for studying central antinociceptive activity [40].
It has been reported that steroidal saponins are among the major chemical constituents in medicinal preparations responsible for most of the anti-inflammatory and analgesic activities. Recent reports also indicate that saponins suppress the expression of iNOS and COX-2, thus resulting in a noticeable lowering of prostaglandin E2 levels [41,42]. The phytochemical analysis of T. govanianum rhizomes showed that it is a rich source of steroids and saponins (Figure 1) [36], therefore it is assumed that the observed effects are due to the presence of these metabolites in T. govanianum [43,44].
It is worth mentioning that mice were selected as the species of choice in these specific tests because they are noticeably sensitive not only to opioid-mediated effects, but also to coexistent non-steroidal anti-inflammatory drugs (NSAIDs) activity [45,46]. Moreover, the murine abdominal constriction assays as well as the hot plate test detect dose-dependent anti-nociception in this species quite well [47,48].
3. Experimental Section
3.1. Plant Material
T. govanianum rhizomes were collected from Khyber Pakhtunkhwa, Upper Dir, Kohistan valley (34°54′ and35° 52′ North latitudes, 72°43′ and 73°57′ East longitudes), in August, 2013. The plant was authenticated by Mr. Ghulam Jelani (Curator) of the Department of Botany, University of Peshawar. Afterwards, a voucher specimen [Bot. 20092 (PUP)] of this plant was deposited in the herbarium for record.
3.2. Extraction and Fractionation
The shade-dried rhizomes of T. govanianum (7 kg) were grounded and extracted with MeOH (40 L) at room temperature, three times for seven days (3 × 40 L). The combined methanolic extract was evaporated to dryness that yielded a brownish gummy residue (512 g). It was further fractionated (solid-liquid partition) into hexane (Hex-fr, 81 g), chloroform (Chl-fr, 94 g), ethyl acetate (EtOAc-fr, 85 g), n-butanol (BuOH-fr, 115 g), and aqueous (Aq-fr, 205 g) fractions. The chloroform and n-butanol fractions were subjected to column chromatography and elution was carried out with mixture of hexane, chloroform, EtOAc and MeOH in increasing order of polarity. The compounds diosgenin and pennogenin were obtained at 20 and 25% EtOAc/hexane solvent system, respectively, while borassoside was obtained at 5% MeOH/EtOAc solvent system. The doses of the extract and its fractions used in the in vivo assays were selected in relation to the usual therapeutic doses of the respective standard drugs (controls) utilized in the pharmacological activities.
3.3. Animals
BALB/c mice of either sex (21–35 g) were maintained and acclimatized at 25 ± 2 °C under a 12 h dark/light cycle. Food and water were provided ad libitum. The experimental procedures were approved by the Ethical Committee of the Department of Pharmacy, University of Peshawar, Pakistan (14/EC/Pharm).
3.4. Acute Toxicity Test
3.4.1. In Vivo Toxicity
The acute toxicity test was performed according to the organization for economic co-operation for development (OECD) guidelines for the testing of chemicals, Test No. 423 (OECD guidelines, 2001) and the same methodology is also used for screening of medicinal plants [49]. The animals were divided into six groups each consisting of six mice (n = 6). T. govanianum extract was administered in doses of 250, 500, 1000, 1500, 3000 and 6000 mg/kg body weight through an oral gavage tube and the morbidity was observed continuously for the first 2 h and mortality up to 24 h post dose administration [50]. The animals were observed for spontaneous activity, aggressiveness, cyanosis, ataxia, tail pinch response, righting reflex, writhing, convulsions, catalepsy and bizarre behavior. The 50% mortality among the animals indicated the toxicity concentration of the extract which was calculated using probit analysis.
3.4.2. In Vitro Toxicity
The in vitro cytotoxic activity of methanol extract and fractions thereof was determined by following MTT assay, with slight modification [51,52], on 3T3 cell lines.
3.5. Anti-Inflammatory Assay
3.5.1. Carrageenan Induced Paw Edema Assay
The anti-inflammatory activity was performed using the carrageenan induced paw edema model in mice [53]. Briefly, BALB/c mice (25–30 g) were randomly divided into five groups (n = 6). Group I was treated with normal saline (10 mL/Kg), group II with diclofenac sodium (10 mg/Kg), while the other groups were treated with T. govanianum rhizomes MeOH-ext and its fractions (50, 100 and 200 mg/Kg; p.o.). After 30 min of drug treatment, 0.05 mL low molecular weight carrageenan (MW 20–30 kD, 1% w/v in 0.9% NaCl w/v solution) was injected subcutaneously in the sub plantar tissue of the right hind paw of each mouse. The inflammation was measured using a digital plethysmometer (LE 7500 plan lab S.L) immediately after injection of carrageenan and, then, at 1, 2, 3, 4 and 5 h intervals. The average paw swelling in tested samples as well as standard treated animals was compared with that of control and percent inhibition (anti-inflammatory activity) of edema was determined using the following formula:
| Percentage of edema inhibition (%) = (VA − VB/VA) × 100 |
where VA is the edema volume of control and VB is the volume of paw edema in treated group.
3.5.2. Chemiluminescence Assay
Luminol-enhanced chemiluminescence assay was performed, as per previously reported method [54]. In brief, 25 µL of diluted whole blood HBSS++ (Hanks Balanced Salt Solution, containing calcium chloride and magnesium chloride) (Sigma, St. Louis, MO, USA) was incubated with 25 µL of three different concentrations of test compounds (1, 10 and 100 µg/mL), each in triplicate. Control wells received HBSS++ and cells, but no compound. Test was performed in white half area 96 well plates (Costar, New York, NY, USA), which was incubated at 37 °C for 15 min in the thermostat chamber of luminometer (Labsystems, Helsinki, Finland). After incubation, 25 µL of serum opsonized zymosan (SOZ) (Fluka, Buchs, Switzerland) and 25 µL of intracellular reactive oxygen species detecting probe, luminol (Research Organics, Cleveland, OH, USA), were added into each well, except blank wells (containing only HBSS++). The level of ROS was recorded by a luminometer and expressed as relative light units (RLU). Drug ibuprofen was used as positive control.
3.6. Analgesic Activity
3.6.1. Abdominal Constriction Assay
The acetic acid induced abdominal constriction assay was performed to determine the peripheral antinociceptive effect [47]. The animals (mice) were withdrawn from food 2 h before the start of experiment. T. govanianum rhizomes extract and fractions thereof were administered orally through an oral gavage tube at doses of 50 and 100 mg/kg. Diclofenac sodium was used as standard and was administered at a dose of 50 mg/kg, (i.p.). After 1 h of test drug and standard drug treatments, all animals were injected with 1% acetic acid, (i.p.). The numbers of writhes were counted after 5 min of acetic acid injection up to 20 min.
3.6.2. Hot Plate Test
The central analgesic effect of T. govanianum rhizomes was evaluated by using hot plate test method [55]. Animals (mice) were withdrawn from food 2 h prior to experiment. All animals were screened for pre-test latency time and only those animals having a pre-test latency time of <15 s were selected for experiment. A cut off time, 30 s was set to avoid any thermal injury. T. govanianum rhizomes extract and its fractions were administered orally through an oral gavage tube at doses of 50 and 100 mg/kg. Tramadol served as positive control and was administered at a dose of 30 mg/kg, (i.p.). After 1 h of extract/fractions and 30 min of standard drug administration, the latency time was measured at 30, 60, 90 and 120 min using a hot plate (Harvard apparatus, Kent, UK) maintained at 54 ± 0.1 °C.
3.7. Statistical Analysis
Animals were randomly assigned to each treatment groups. Results are presented as mean ± SD or SEM. Statistical comparisons were carried out by one way ANOVA followed by Dunnett’s, post-hoc test using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). The LD50 value in the acute toxicity test was determined by Probit analysis using Minitab version 17.1.0 (Minitab Inc., State College, PA, USA). A p value of 0.05 was considered as significant.
4. Conclusions
The traditional medicinal use of T. govanianum rhizomes to treat pain and inflammation can be validated by findings in our study, where the extract, its fractions and isolated compounds afforded selective antinociceptive and anti-inflammatory properties in animal models. Therefore, T. govanianum rhizomes may serve as a potential source of novel compounds effective in pain and inflammatory conditions.
Acknowledgments
The authors are grateful to the H.E.J. Research Institute of Chemistry, University of Karachi, Pakistan, for providing necessary facilities.
Abbreviations
| MeOH-ext | methanol extract |
| Chl-fr | chloroform fraction |
| EtOAc-fr | ethyl acetate fraction |
| BuOH-fr | n-butanol fraction |
| Hex-fr | n-hexane fraction |
| Aq-fr | aqueous fraction |
| COX-2 | cyclooxygenase-2 |
| HBSS++ | Hanks balanced salt solution |
| iNOS | inducible nitric oxide synthase |
| RLU | relative light units |
| ROS | reactive oxygen species |
| SOZ | serum opsonized zymosan |
| PG | prostaglandins |
| NO | nitric oxide |
| HOCl | hypochlorous acid |
| SOD | superoxide dismutase |
| IL | interleukin |
Supplementary Materials
The supplementary materials are available online at: http://www.mdpi.com/1420-3049/21/8/1095/s1.
Author Contributions
Shafiq Ur Rahman and Marcello Iriti conceived, designed and carried out experiments. Achyut Adhikari Muhammad Raza Shah, Muhammad Khurram, Muhammad Ismail, and Fazal Akbar interpreted the results and assisted in manuscript preparation. Muhammad Shahid, Abdul Haseeb and Farman Ali performed statistical analysis and prepared the figures. All authors read and approved the paper before its final submission.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Munuswamy H., Thirunavukkarasu T., Rajamani S., Elumalai E.K., Ernest D. A review on antimicrobial efficacy of some traditional medicinal plants in Tamilnadu. J. Acute Dis. 2013;2:99–105. doi: 10.1016/S2221-6189(13)60107-9. [DOI] [Google Scholar]
- 2.Patwardhan B., Warude D., Pushpangadan P., Bhatt N. Ayurveda and traditional Chinese medicine: A comparative overview. Evid. Based. Complement. Altern. Med. 2005;2:465–473. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ficker C.E., Smith M.L., Susiarti S., Leaman D.J., Irawati C., Arnason J.T. Inhibition of human pathogenic fungi by members of Zingiberaceae used by the Kenyah (Indonesian Borneo) J. Ethnopharmacol. 2003;85:289–293. doi: 10.1016/S0378-8741(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 4.Ficker C., Smith M., Akpagana K., Gbeassor M., Zhang J., Durst T., Assabgui R., Arnason J. Bioassay guided isolation and identification of antifungal compounds from ginger. Phytother. Res. 2003;17:897–902. doi: 10.1002/ptr.1335. [DOI] [PubMed] [Google Scholar]
- 5.Yokosuka A., Kawakami S., Haraguchi M., Mimaki Y. Seven new triterpene glycosides from the pericarps of Stryphnodendron fissuratum. Phytochem. Lett. 2011;4:259–266. doi: 10.1016/j.phytol.2011.04.010. [DOI] [Google Scholar]
- 6.Gupta S. Prospects and perspectives of natural plants products in medicine. Indian. J. Pharmacol. 1994;26:1–12. [Google Scholar]
- 7.Ahmad F., Rafeeq A.K., Shahid R. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. J. Islam. Acad. Sci. 1992;5:111–114. [Google Scholar]
- 8.Dina T.A., Rahman M.A., Ahmed N.U., Uddin M.N. Analgesic and anti-inflammatory properties of Argyreia argentea methanol extract in animal model. J. Taibah Univ. Sci. 2010;3:1–7. doi: 10.1016/S1658-3655(12)60014-4. [DOI] [Google Scholar]
- 9.Muhammad S.K. Ph.D. Thesis. Quaid-i-Azam University; Islamabad, Pakistan: 2011. [(accessed on 21 May 2016)]. Diversity of Vascular Plants, Ethnobotany and Conservation Status of Ushairy Valley, District Dir, Upper NWFp Northern Pakistan. Available online: http://eprints.hec.gov.pk/7497/ [Google Scholar]
- 10.Mahmood A., Mahmood A., Malik R.N., Shinwari Z.K. Indigenous knowledge of medicinal plants from Gujranwala district, Pakistan. J. Ethnopharmacol. 2013;148:714–723. doi: 10.1016/j.jep.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Pant S., Samant S. Ethnobotanical observations in the Mornaula reserve forest of Komoun, West Himalaya, India. Ethnobot. Leafl. 2010;14:193–217. [Google Scholar]
- 12.Rani S., Rana J., Rana P. Ethnomedicinal plants of Chamba district, Himachal Pradesh, India. J. Med. Plant Res. 2013;7:3147–3157. [Google Scholar]
- 13.Gates R.R. A systematic study of the North American genus Trillium, its variability, and its relation to Paris and Medeola. Ann. MI. Bot. Gard. 1917;4:43–92. doi: 10.2307/2990062. [DOI] [Google Scholar]
- 14.Yokosuka A., Mimaki Y. Steroidal glycosides from the underground parts of Trillium erectum and their cytotoxic activity. Phytochemistry. 2008;69:2724–2730. doi: 10.1016/j.phytochem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Huang W., Zou K. Cytotoxicity of a plant steroidal saponin on human lung cancer cells. Asian Pac. J. Cancer Prev. 2011;12:513–517. [PubMed] [Google Scholar]
- 16.Ono M., Sugita F., Shigematsu S., Takamura C., Yoshimitsu H., Miyashita H., Ikeda T., Nohara T. Three new steroid glycosides from the underground parts of Trillium kamtschaticum. Chem. Pharm. Bull. 2007;55:1093–1096. doi: 10.1248/cpb.55.1093. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Liu C., Xiao D., Han J., Yue Z., Sun Y., Fan L., Zhang F., Meng J., Zhang R. Trillium tschonoskii steroidal saponins suppress the growth of colorectal cancer cells in vitro and in vivo. J. Ethnopharmacol. 2015;168:136–145. doi: 10.1016/j.jep.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 18.Huang W., Zou K., Xiong B. The rhizome of Trillium tschonoskii Maxim. Extract induces apoptosis in human lung cancer cells. Z. Naturforsch. A Phys. Sci. 2011;66:477–484. doi: 10.5560/ZNC.2011.66c0477. [DOI] [PubMed] [Google Scholar]
- 19.Fu L.K., Jin J.M. China Plant Red Data Book-Rare and Endangered Plants. Science Press; Beijing, China: 1992. [Google Scholar]
- 20.Wang J., Zou K., Zhang Y., Liu C., Wu J., Zhou Y., Dan F., Zhang Y. An 18-norspirostanol saponin with inhibitory action against COX-2 production from the underground part of Trillium tschonoskii. Chem. Pharm. Bull. 2007;55:679–681. doi: 10.1248/cpb.55.679. [DOI] [PubMed] [Google Scholar]
- 21.Yu L.l., Zou K., Wang J.Z., Zhu L.B., Zhou Y., Yang J. Study on the anti-inflammatory, analgesic and thrombosis effects of extract of Trillium tschonoskii Maxim. Lishizhen Med. Mater. Med. Res. 2008;19:1178–1180. [Google Scholar]
- 22.Deni B. The Royal Horticultural Society Encyclopedia of Herbs and Their Uses. Dorling Kindersley B. 1996;424:116–122. [Google Scholar]
- 23.Rahman S.U., Ismail M., Shah M.R., Adhikari A., Anis I., Ahmad M.S., Khurram M. Govanoside A, a new steroidal saponin from rhizomes of Trillium govanianum. Steroids. 2015;104:270–275. doi: 10.1016/j.steroids.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Rahman S.U., Ismail M., Khurram M., Haq I.U. Pharmacognostic and ethnomedicinal studies on Trillium govanianum. Pak. J. Bot. 2015;47:187–192. [Google Scholar]
- 25.Rahman S.U., Ismail M., Shah M.R., Iriti M., Shahid M. GC/MS analysis, free radical scavenging, anticancer and β-glucuronidase inhibitory activities of Trillium govanianum rhizomes. Bangladesh J. Pharmacol. 2015;10:577–583. doi: 10.3329/bjp.v10i3.23446. [DOI] [Google Scholar]
- 26.Pawliczak R. The role of radical oxygen species in airway inflammation. Pol. Merkur. Lekarski. 2003;14:493–496. [PubMed] [Google Scholar]
- 27.Cuzzocrea S., Mazzon E., Dugo L., Serraino I., Ciccolo A., Centorrino T., De Sarro A., CAPUTI A.P. Protective effects of n-acetylcysteine on lung injury and red blood cell modification induced by carrageenan in the rat. FASEB J. 2001;15:1187–1200. doi: 10.1096/fj.00-0526hyp. [DOI] [PubMed] [Google Scholar]
- 28.Salvemini D., Wang Z.-Q., Bourdon D.M., Stern M.K., Currie M.G., Manning P.T. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J. Pharmacol. 1996;303:217–220. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 29.Fridovich I. Superoxide anion radical (O2), superoxide dismutases, and related matters. J. Biol. Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 30.Uttara B., Singh A.V., Zamboni P., Mahajan R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sultana N., Arayne M.S., Naz A., Mesaik M.A. Identification of anti-inflammatory and other biological activities of 3-carboxamide, 3-carbohydrazide and ester derivatives of gatifloxacin. Chem. Cent. J. 2013;7:6. doi: 10.1186/1752-153X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gene R.M., Segura L., Adzet T., Marin E., Iglesias J. Heterotheca inuloides: Anti-inflammatory and analgesic effect. J. Ethnopharmacol. 1998;16:157–162. doi: 10.1016/S0378-8741(97)00155-4. [DOI] [PubMed] [Google Scholar]
- 33.Salvemini D., Wang Z.Q., Wyatt P.S., Bourdon D.M., Marino M.H., Manning P.T., Currie M.G. Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ianaro A., O’donnell C., Di Rosa M., Liew F. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology. 1994;82:370–375. [PMC free article] [PubMed] [Google Scholar]
- 35.Seibert K., Zhang Y., Leahy K., Hauser S., Masferrer J., Perkins W., Lee L., Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fantone J.C., Ward P. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 1982;107:395–418. [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W.H., An-Rong L., Ching-Huey Y. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci. Biotechnol. Biochem. 2006;70:2371–2380. doi: 10.1271/bbb.50698. [DOI] [PubMed] [Google Scholar]
- 38.Punitha D., Udhayasankar M.R., Danya U., Arumugasamy K., Shalimol A. Anti-inflammatory Activity of Characterized Compound Diosgenin Isolated from Tinospora malabarica Miers in Ann. (Menispermaceae) in Animal Model. Int. J. Herb. Med. 2013;1:76–78. [Google Scholar]
- 39.Ferreira S., Lorenzetti B.B., Castro M.S.A., Correa F. The Recognition of Anti-rheumatic Drugs. MTP Press Ltd.; Lancaster, PA, USA: 1978. Antialgic effect of aspirin-like drugs and the inhibition of prostaglandin synthesis; pp. 25–37. [Google Scholar]
- 40.Hosseinzadeh H., Younesi H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J.-R., Zhou H., Jiang Z.-H., Wong Y.F., Liu L. In vivo anti-inflammatory and analgesic activities of a purified saponin fraction derived from the root of Ilex pubescens. Biol. Pharm. Bull. 2008;31:643–650. doi: 10.1248/bpb.31.643. [DOI] [PubMed] [Google Scholar]
- 42.Navarro P., Giner R.M., Recio M.C., Máñez S., Cerdá-Nicolás M., Ríos J.-L. In vivo anti-inflammatory activity of saponins from Bupleurum rotundifolium. Life Sci. 2001;68:1199–1206. doi: 10.1016/S0024-3205(00)01019-5. [DOI] [PubMed] [Google Scholar]
- 43.Shao B., Hongzhu G., Yajun C., Min Y., Han J., Guo D. Steroidal saponins from Smilax china and their anti-inflammatory activities. Phytochemistry. 2007;68:623–630. doi: 10.1016/j.phytochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Agnihotri S., Wakode S., Agnihotri A. An overview on anti-inflammatory properties and chemo-profiles of plants used in traditional medicine. Indian J. Nat. Prod. Resour. 2010;1:150–167. [Google Scholar]
- 45.Boué J., Blanpied C., Brousset P., Vergnolle N., Dietrich G. Endogenous opioid-mediated analgesia is dependent on adaptive T cell response in mice. J. Immunol. 2011;186:5078–5084. doi: 10.4049/jimmunol.1003335. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura H., Shimoda A., Ishii K., Kadokawa T. Central and peripheral analgesic action of non-acidic non-steroidal anti-inflammatory drugs in mice and rats. Arch. Int. Pharmacodyn. Ther. 1986;282:16–25. [PubMed] [Google Scholar]
- 47.Collier H., Dinneen L., Johnson C.A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjølsen A., Rosland J.H., Berge O.-G., Hole K. The increasing-temperature hot-plate test: An improved test of nociception in mice and rats. J. Pharmacol. Methods. 1991;25:241–250. doi: 10.1016/0160-5402(91)90014-V. [DOI] [PubMed] [Google Scholar]
- 49.Thangaraj P. Pharmacological Assays of Plant-Based Natural Products. Springe Nature; Basel, Switzerland: 2016. [DOI] [Google Scholar]
- 50.Khan H., Saeed M., Khan M.A., Dar A., Khan I. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J. Ethnopharmacol. 2010;127:521–527. doi: 10.1016/j.jep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Baydoun E., Bibi M., Iqbal M.A., Atia-tul W., Farran D., Smith C., Sattar S.A., Rahman A.U., Choudhary M.I. Microbial transformation of anticancer steroid exemestane and cytotoxicity of its metabolites against cancer cell lines. Chem. Cent. J. 2013;7 doi: 10.1186/1752-153X-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 53.Morris C.J. Carrageenan-induced paw edema in the rat and mouse. Inflamm. Protoc. 2003;225:115–121. doi: 10.1385/1-59259-374-7:115. [DOI] [PubMed] [Google Scholar]
- 54.Helfand S.L., Werkmeister J., Roder J.C. Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. J. Exp. Med. 1982;156:492–505. doi: 10.1084/jem.156.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Callaghan J.P., Holtzman S.G. Quantification of the analgesic activity of narcotic antagonists by a modified hot-plate procedure. J. Pharmacol. Exp. Ther. 1975;192:497–505. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.