Abstract
In recent years, several synthetic strategies aiming at the peripheral functionalization of porphyrins were developed. Particularly interesting are those involving the modification of β-pyrrolic positions leading to pyrrole-modified porphyrins containing four-, five-, six- or seven-membered heterocycles. Azeteoporphyrins, porpholactones and morpholinoporphyrins are representative examples of such porphyrinoids. These porphyrin derivatives have recently gained an increasing interest due to their potential application in PDT, as multimodal imaging contrast agents, NIR-absorbing dyes, optical sensors for oxygen, cyanide, hypochlorite and pH, and in catalysis.
Keywords: porphyrinoids, secochlorins, chlorophins, bacteriophins, azeteoporphyrins, porpholactones, pyriporphyrins, morpholinoporphyrins
1. Introduction
Porphyrins and porphyrinoid compounds, including contracted and expanded porphyrin derivatives, are highly versatile compounds, both in terms of chemistry (which is extremely rich) and diversity of (potential) applications. These two strands have driven the continuous development of new routes to the synthesis of such compounds.
Porphyrin derivatives are being used in biomedical and environmental applications, in catalysis, and in a range of technical applications [1,2,3,4,5]. Most of these applications require compounds that display absorption bands in the 600–800 nm region. Since “simple” porphyrins hardly fulfill this requirement, chlorins, bacteriochlorins, π-extended porphyrins, pyrrole-modified porphyrins, and other porphyrinoids, are a better choice. Such compounds are typically prepared by: (i) the structural modification of already existing porphyrins via, for instance, cycloaddition reactions, electrophilic or nucleophilic aromatic substitutions, pyrrole ring-contraction or -expansion reactions; or (ii) by constructing the porphyrin macrocycle using adequate pyrrolic building blocks. Considering the last approach, the “3 + 1 method” was extensively used for the synthesis of chlorins and pyrrole-modified porphyrins. This subject has been reviewed recently [6,7,8,9] and will not be covered here. The metallation of porphyrinoids has also been covered in recent reviews [10,11,12].
The modification of the periphery of porphyrins using cycloaddition reactions, namely Diels-Alder reactions and 1,3-dipolar cycloadditions, is a remarkable method to produce chlorins, bacteriochlorins or isobacteriochlorins [13,14,15]. We and other groups have reported several works using porphyrins as dienophiles in Diels–Alder reactions [16,17,18,19,20,21] or as dipolarophiles in 1,3-dipolar cycloadditions [22,23,24,25,26,27,28,29,30,31].
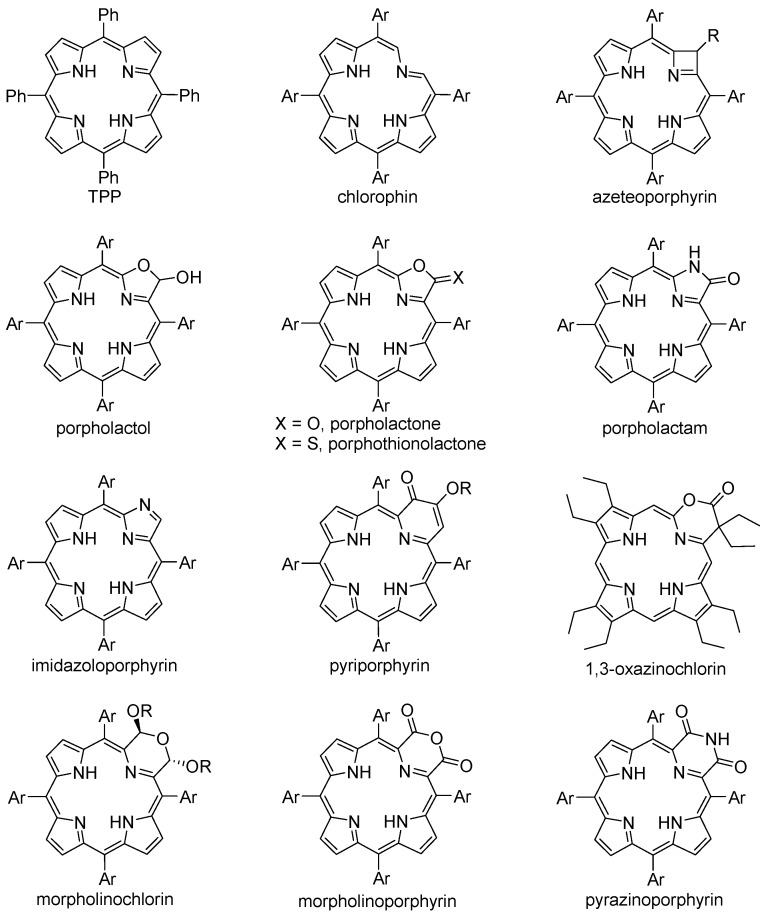
Concerning the transformation of porphyrins into pyrrole-modified porphyrinoids, many pyrrole ring-contraction and -expansion reactions were reported during the last three decades. However, a substantial part of that work was published in recent years by the group of Brückner that, by using the “breaking and mending of porphyrins” approach [32], was able to produce an enormous diversity of pyrrole-modified porphyrins (some are exemplified in Figure 1).
Figure 1.
meso-Tetraphenylporphyrin (TPP) (for comparison) and examples of pyrrole-modified porphyrins discussed in this review.
This review covers the reported methods for the modification of the porphyrin macrocycle and the potential applications of the resulting porphyrinoid compounds. This subject has already been discussed in recent reviews [33,34].
2. Chemistry
The conversion of a porphyrin, or a metalloporphyrin, into a pyrrole-modified porphyrinoid is frequently a multi-step process that requires the separation, purification and structural characterization of the intermediate compounds. However, in this article only the final steps of such transformations are discussed. The methods described below were organized according to the types of porphyrin derivatives used as immediate precursors of pyrrole-modified porphyrinoids.
2.1. From N-Substituted Porphyrins
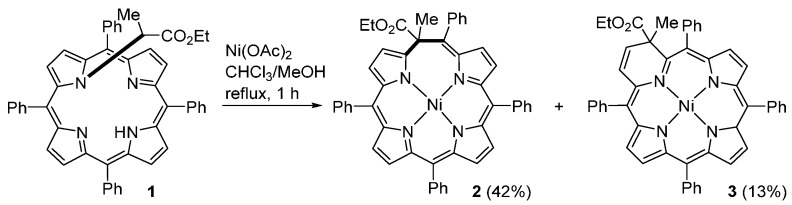
The metallation of the N-substituted porphyrin 1 with nickel acetate in a refluxing CHCl3/MeOH mixture leads to the formation of two new porphyrinoids: the expanded porphyrin 2 and the pyriporphyrin 3 (Scheme 1) [35,36]. Compounds 2 and 3 result from the insertion of a C atom in an α–meso bond or in an α–β bond, respectively. This reaction, reported by Callott and Schaeffer in 1978, is one of the first examples of the direct conversion of a porphyrin into a pyrrole-modified porphyrin.
Scheme 1.
Synthesis of an expanded porphyrin and a pyriporphyrin [35,36].
2.2. From β-Aminoporphyrins
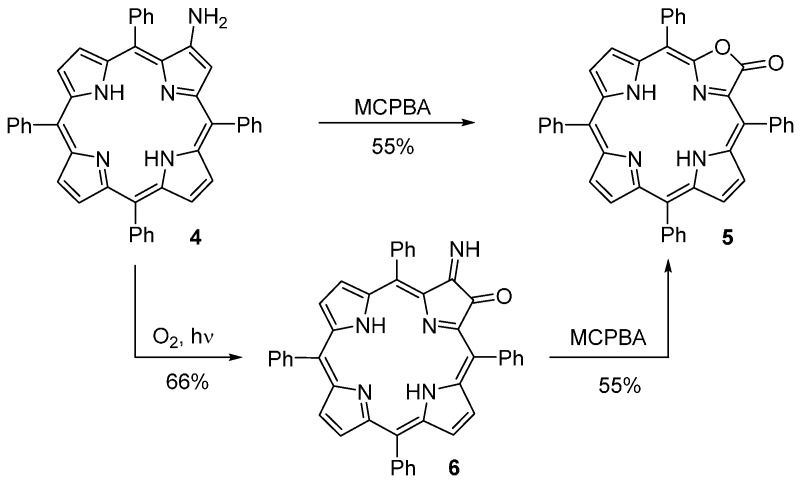
In 1984, Crossley and King [37,38] found that the oxidation of β-amino-meso-tetraphenyl-porphyrin (4) with m-chloroperbenzoic acid (MCPBA) affords porpholactone 5 in 55% yield (Scheme 2). The same porpholactone can be obtained in similar yield by oxidation of the imino-oxochlorin 6 (obtained by photo-oxidation of 2-aminoporphyrin 4). In their initial communication, these authors also reported the synthesis of a ring-expanded morpholinoporphyrin derivative and a ring-contracted azeteoporphyrin (see Scheme 15).
Scheme 2.
Routes to meso-tetraphenylporpholactone reported by Crossley and King [37].
Scheme 15.
Route to 4- and 6-membered porphyrinoids [37].
The method reported by Crossley and King (involving β-nitration of meso-tetraarylporphyrins, followed by reduction and oxidation of the resulting β-aminoporphyrins with MCPBA) was used by other groups to prepare porpholactones and the corresponding iron complexes [39].
2.3. From 2,3-Dihydroxychlorins
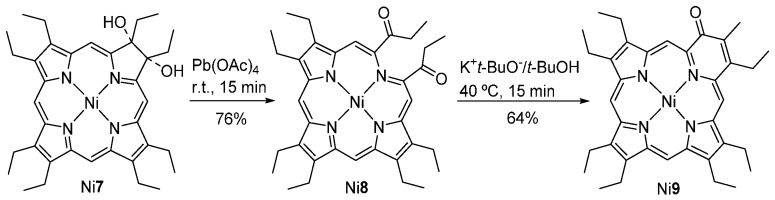
In 1993, Bonnett and co-workers [40,41] reported that the oxidative cleavage of the nickel(II) dihydroxychlorin Ni7 with lead tetraacetate, at room temperature, affords the secochlorin diketone Ni8 in 76% yield (Scheme 3) [42]. Treatment of Ni8 with potassium tert-butoxide in tert-butyl alcohol at 40 °C leads to the formation of the pyridone-modified porphyrin Ni9 in 64% yield (via an intramolecular aldol condensation). Pb(OAc)4 cannot be used in the oxidation of the free-base 7 since it does not allow the isolation of any product in reasonable yield.
Scheme 3.
Route to the pyridone-modified porphyrin Ni9 reported by Bonnett and co-workers [40,41].
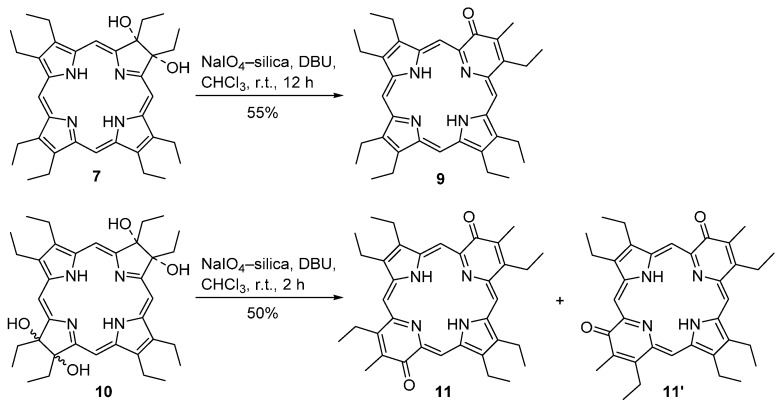
Aiming to synthesize free-base pyriporphyrins, Brückner and co-workers used a slurry of silica gel–NaIO4 in CHCl3 in the presence of 5–10 vol % DBU to convert the free-base dihydroxychlorin 7 to pyriporphyrin 9 (Scheme 4) [43]. This product could be isolated in 55% yield (after chromatographic separation and crystallization) as a purple microcrystalline solid. This method was also applied to the conversion of the tetrahydroxybacteriochlorin 10 into the isomeric bis(pyri)porphyrins 11 and 11’, which were isolated in a combined yield of 50%.
Scheme 4.
Route to the pyridone-modified porphyrins reported by Brückner and co-workers [43].
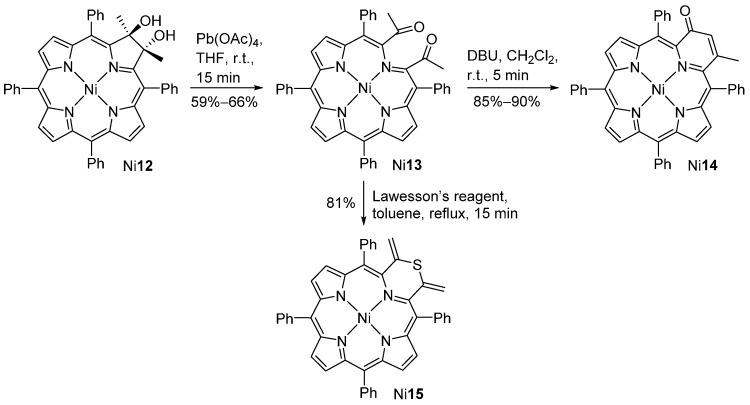
Starting from the trans-2,3-dimethyl-2,3-dihydroxychlorin Ni12, and using lead tetraacetate for the oxidative diol cleavage, Brückner and co-workers were able to generate the 2,3-diacetylsecochlorin Ni13 (Scheme 5) [44]. Under Brønsted-basic conditions, this diketone cyclizes via an intramolecular aldol condensation to provide the pyriporphyrin derivative Ni14. Reaction of the 2,3-diacetylsecochlorin Ni13 with Lawesson’s reagent induces the formation of the thiomorpholinochlorin Ni15 substituted with two methylene groups.
Scheme 5.
Routes to pyridone- and thiomorpholine-modified porphyrins [44].
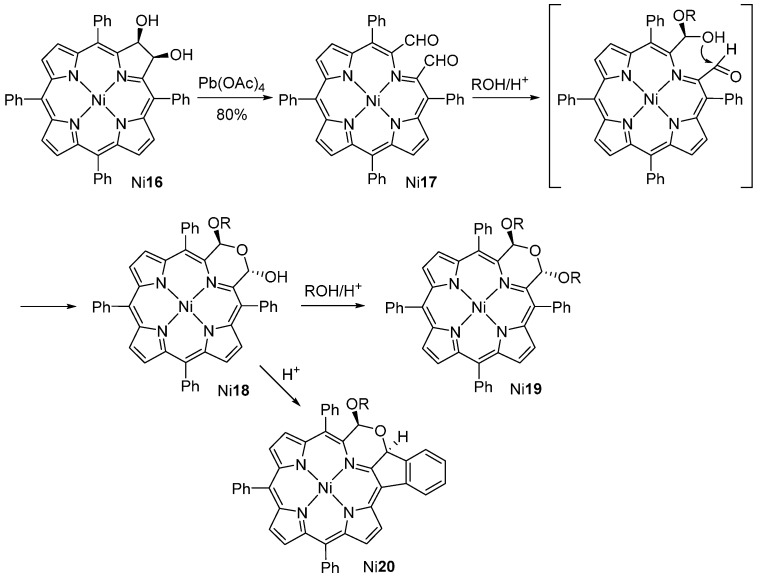
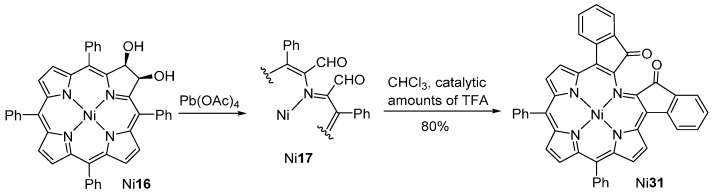
Brückner and co-workers [45,46] also used a 2,3-dihydroxychlorin as an intermediate in the synthesis of porphyrinoids in which one pyrrolic unit is formally replaced by a morpholine ring (Scheme 6). The 2,3-dihydroxy-meso-tetraphenylchlorin Ni16, obtained by osmium tetroxide-mediated dihydroxylation [47,48] of meso-tetraphenylporphyrin (TPP) and complexation with nickel acetate, was transformed into the secochlorin-2,3-dicarbaldehyde Ni17 by oxidation with lead tetraacetate. The dicarbaldehyde Ni17 undergoes intramolecular acetal formation when treated with alcohols in the presence of acid to produce a mixture of morpholinochlorins Ni18 and Ni19 (see also Scheme 11). Acid treatment of hydroxymorpholinochlorins Ni18 leads to the establishment of an intramolecular β-to-o-phenyl linkage resulting in the formation of the polycyclic-fused porphyrin system Ni20 [49,50]. Complexes of types Ni18, Ni19 and Ni20 (and most of their free-bases) exhibit a ruffled macrocycle with an inherent helical chirality. The resolution of the racemic mixtures can be achieved, both by classical methods via diastereomers or by HPLC on a chiral phase [49,50].
Scheme 6.
Route to morpholinochlorins [45].
Scheme 11.
Synthesis of indaphyrins [62].
A electrochemical study of Ni(II) porphyrinoids showed that, upon electrochemical reduction, morpholinochlorins form ligand-based reduction products while the conformationally flexible chlorin and secochlorin complexes form Ni(I) complexes [51].
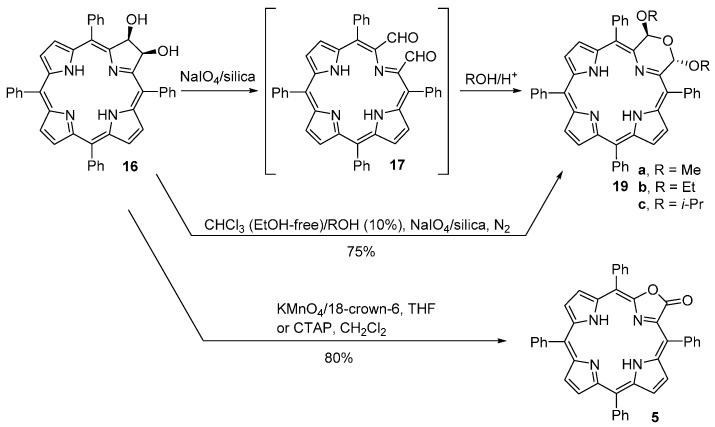
The group of Brückner also reported a variation of the previous method that allowed the synthesis of free-base morpholinochlorins and porpholactones (Scheme 7) [52]. Using the free-base dihydroxychlorin 16 and NaIO4 (heterogenized on silica gel) as the oxidant, this group was able to produce, isolate and characterize the unstable secochlorin-2,3-dicarbaldehyde 17. Reaction of 17 with MeOH, EtOH or i-PrOH under acid catalysis provided the stable morpholinochlorins 19a–c. The same compounds can be obtained directly from the reaction of 16 with NaIO4/silica under N2 in the presence of the corresponding alcohol. Treatment of methoxy derivative 19a with excess EtOH or i-PrOH under acid catalysis at 65 °C leads to alkoxy exchange and formation of 19b or 19c, respectively [52]. MnO4-induced cleavage of diol 16 under phase transfer catalysis (or using cetyltrimethylammonium permanganate, CTAP) affords porpholactone 5 in overall excellent yield (up to 80%). The formation of 5 probably involves the oxidation of the dicarbaldehyde 17 to the corresponding secochlorin-2,3-dicarboxylate followed by decarboxylation and lactonization [52]. This methodology has been used for the synthesis of a range of porpholactones [53,54]. A similar approach has been used for the conversion of meso-tetraphenylporphyrin N-oxide into pyrrole-modified porphyrin N-oxides [55].
Scheme 7.
Route to free-base morpholinochlorins and porpholactones [52].
The group of Brückner demonstrated that Ag(II) can serve as a removable metal template in the oxidative cleavage of 2,3-dihydroxychlorins, allowing an easy access to free-base pyrrole-modified porphyrins [56].
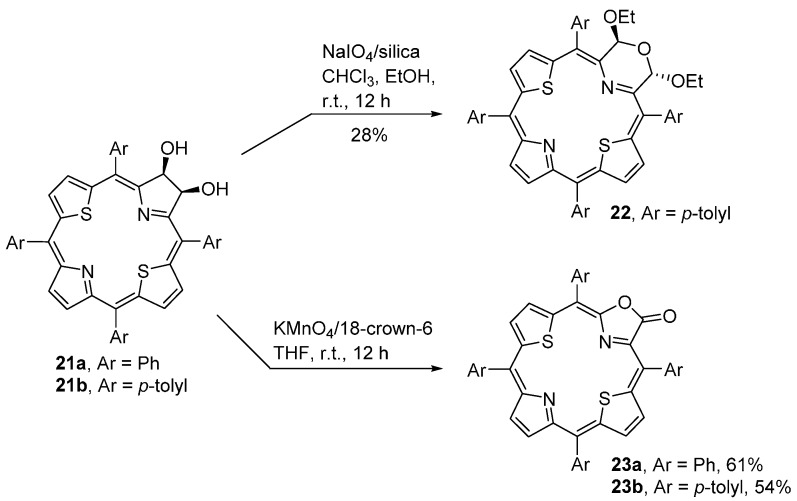
The methods described above were also successfully applied to the conversion of dihydroxy-21,23-dithiachlorins 21a,b into the dithiamorpholinochlorin 22 and dithiaporpholactones 23a,b (Scheme 8) [57,58].
Scheme 8.
Routes to morpholinodithiachlorins and dithiaporpholactones [58].
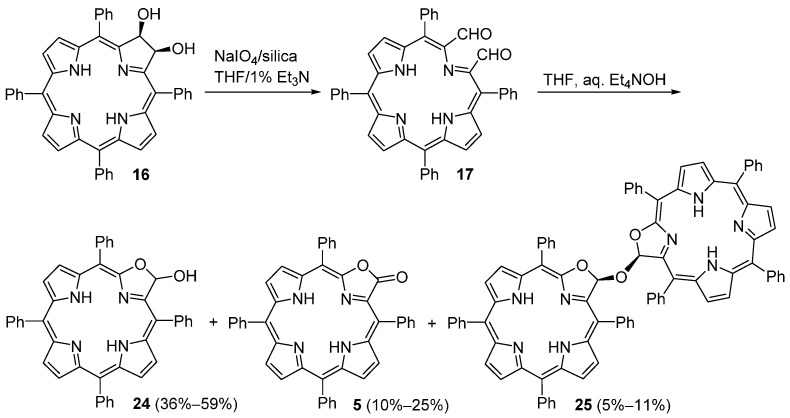
Oxidative cleavage of the purple dihydroxychlorin 16 with NaIO4 heterogenized onto silica gel, in THF containing 1–2 vol % Et3N, converts it in essentially quantitative yields into the unstable free-base secochlorin-2,3-dicarbaldehyde 17 (a brown nonpolar compound) (Scheme 9) [59]. This dialdehyde can be purified by column chromatography (silica gel, CH2Cl2–0.1% Et3N) but in solution, particularly in acidic and/or wet solvents or on silica gel, it tends to decompose within several hours. However, evaporated to dryness and kept in a freezer at −18 °C, it is stable over several months [59]. When a solution of 17 in THF is treated with a large excess of a 30% aqueous solution of Et4NOH, three purple products are formed in varying yields (Scheme 9). The most polar compound (Rf = 0.41, silica–CH2Cl2) is the porpholactol 24, the one with intermediate polarity (Rf = 0.78, silica–CH2Cl2) is the porpholactone 5 and the least polar one (Rf = 0.90, silica–CH2Cl2) is the porpholactol dimer 25 (isolated in yields up to 11%). The authors proposed a mechanism to rationalize the formation of these products which involves an intramolecular Cannizzaro reaction in the dialdehyde 17 [59].
Scheme 9.
Route to free-base oxazolochlorins [59].
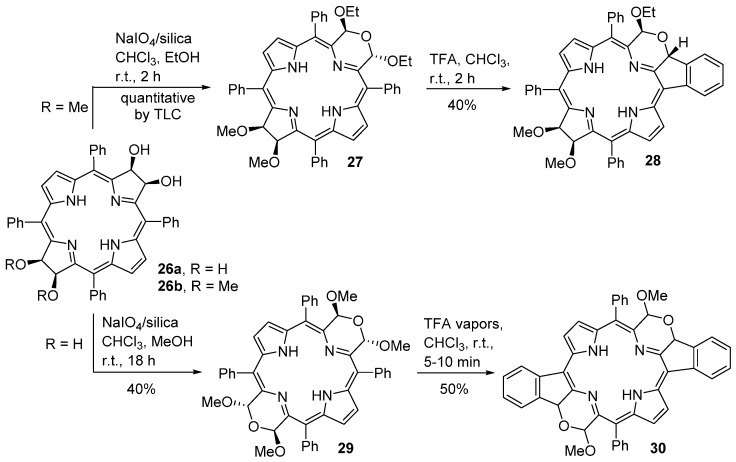
The method described above was also applied to the synthesis of pyrrole-modified bacteriochlorins [60]. Starting from the free-bases 2,3-dihydroxy-12,13-dimethoxychlorin 26b or the 2,3,12,13-tetrahydroxychlorin 26a [61], and using mild oxidation conditions (NaIO4 heterogenized on silica gel, CHCl3, alcohol, room temperature), Brückner and co-workers were able to synthesize the morpholinobacteriochlorin 27 and the bis(morpholino)bacteriochlorin 29, in one pot, in reasonable yields (Scheme 10). Acid treatment of these morpholinochlorins leads to the formation of the polycyclic-fused porphyrin systems 28 and 30.
Scheme 10.
Synthesis of morpholinobacteriochlorins and bis(morpholino)bacteriochlorins [60].
As shown in Scheme 6, the oxidative cleavage of 2,3-dihydroxy-meso-tetraphenylchlorin Ni16 with lead tetraacetate leads to the formation of morpholinochlorins (via the secochlorin-2,3-dicarbaldehyde Ni17). However, depending on the reaction conditions during the ring cleavage reaction, the formyl groups may react with the adjacent o-phenyl positions to establish direct o-phenyl-to-β-linkages [62,63]. The initially formed carbinols oxidize spontaneously to ketones, resulting in the formation of indaphyrin Ni31 in high yield (Scheme 11).
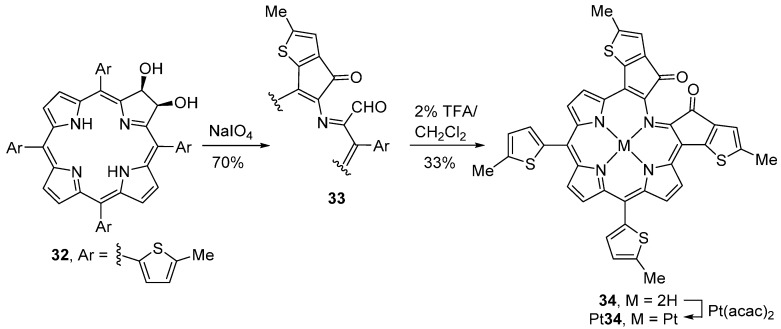
Free-base dihydroxychlorins can also be used to synthesize indaphyrin-type compounds. In that case, the oxidant should be NaIO4 heterogenized on silica gel. As an example, the oxidative cleavage of 2,3-dihydroxy-5,10,15,20-tetrakis(5-methylthien-2-yl)chlorin (32) results in the formation of the monothiaindanone monoaldehyde 33 (presumably via a dicarbaldehyde intermediate) (Scheme 12) [64]. Stirring a solution of monoaldehyde 33 in 2% TFA/CH2Cl2, at room temperature, leads to the formation of thiaindaphyrin 34 in 33% yield. The corresponding platinum(II) complex, Pt34, was obtained in 61% yield from the reaction of 34 with [Pt(acac)2] (3 equiv.) in PhCN for 5 h at reflux temperature [64].
Scheme 12.
Synthesis of thiaindaphyrins [64].
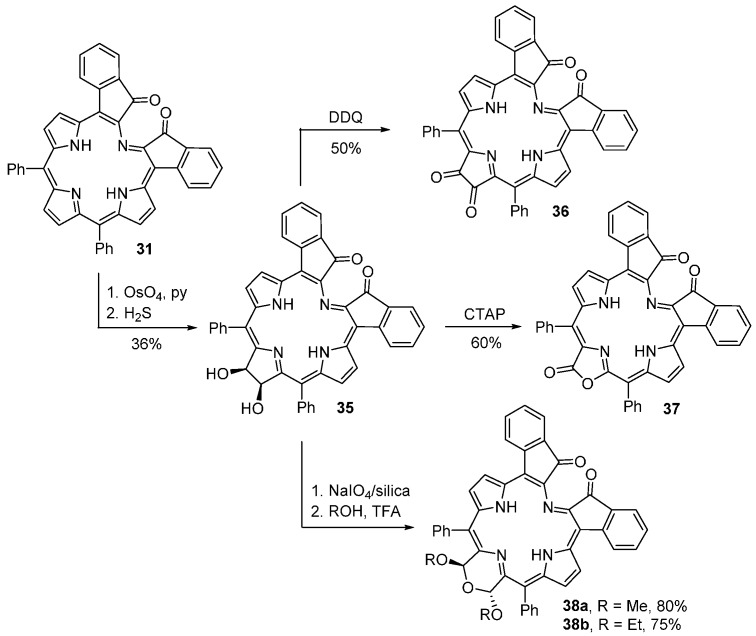
Indaphyrins can be further functionalized to indachlorins, as indicated in Scheme 13 [65]. Using typical porphyrin dihydroxylation conditions, indaphyrin 31 can be converted into the dihydroxylated indachlorin 35. Oxidation of 35 with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) leads to the indachlorin dione 36 while oxidation with CTAP affords the corresponding lactone 37. The dihydroxylated indachlorin 35 can also be converted into dialkoxy-substituted morpholines 38 using standard reaction conditions already described. These compounds display panchromatic absorption spectra between 300 and 900 nm and possess strongly ruffled conformations, incorporating a helimeric twist [65,66]. Resolution of the racemic mixtures of the helimers was achieved by HPLC on a chiral phase and their absolute stereostructures were assigned [66].
Scheme 13.
Conversion of an indaphyrin into indachlorins [65].
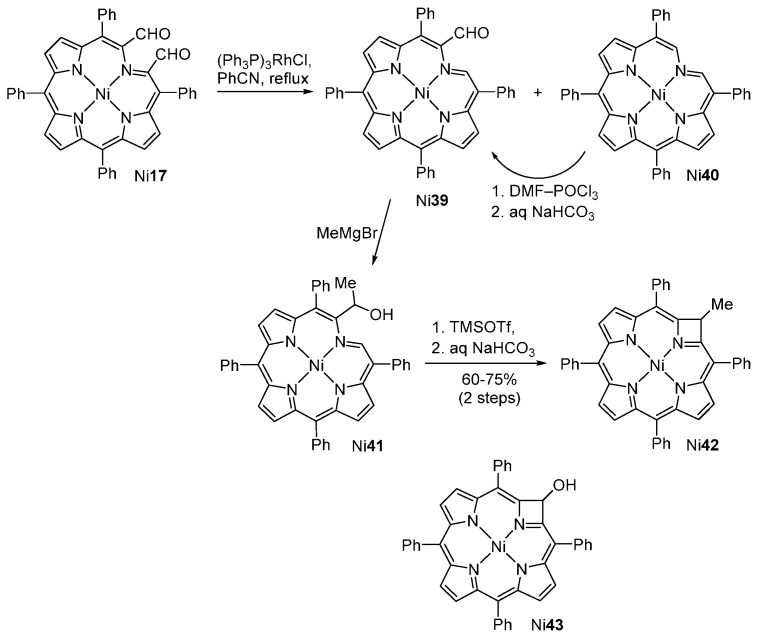
The Brückner group reported a new approach to pyrrole ring-contracted azeteoporphyrins (Scheme 14) [67]. It involves the oxidative cleavage of the dihydroxychlorin Ni16 to the secochlorin-2,3-dicarbaldehyde Ni17 (see Scheme 6) followed by a decarbonylation reaction with an excess of (Ph3P)3RhCl to afford a mixture of the chlorophins Ni39 (12% yield) and Ni40 (60% yield) [46,67]. The monoaldehyde Ni39 reacts with an excess of methylmagnesium bromide leading to the formation of the secondary alcohol Ni41. This alcohol reacts with an excess of TMSOTf to afford the azeteoporphyrin Ni42 in 60%–75% yield after purification by preparative TLC and crystallization. The role of TMSOTf in this cyclization reaction is to induce the removal of the hydroxyl group and generation of the corresponding carbocation. The azete ring is then formed by an intramolecular Friedel-Crafts reaction.
Scheme 14.
Route to azeteoporphyrins [67].
It is interesting to note that, under adequate experimental conditions, alcohol Ni41 is obtained in ca. 7% yield in the decarbonylation reaction of the dicarbaldehyde Ni17 [67]. The existence of alcohol Ni43 had been previously proposed as a side product in the Vilsmeier–Haack formylation of the chlorophin Ni40 [68].
2.4. From 2,3-Dioxochlorins
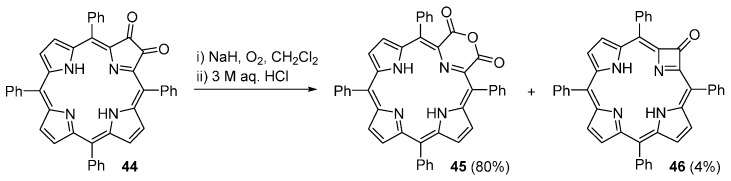
In 1984, Crossley and King reported that treatment of a CH2Cl2 solution of dione 44 (obtained in quantitative yield by acidic hydrolysis of imino-oxochlorin 6) with an excess of NaH and exposed to air, followed by treatment with 3 M aqueous HCl, affords the morpholinoporphyrin 45 in 80% yield (Scheme 15). The ring-contracted azeteoporphyrin 46 is also formed in this reaction as a minor product [37]. Anhydride 45 can also be obtained in 64% yield by treatment of dione 44 with MCPBA [37].
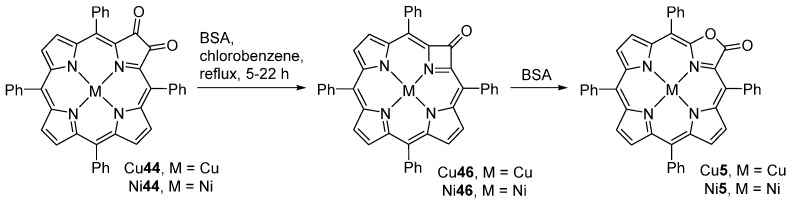
Zaleski and co-workers [69] reported that the Cu(II) and Ni(II) complexes of 2,3-dioxochlorins react with benzeneselenic anhydride (BSA) leading to the formation of ring-contracted azetine derivatives M46 that further react with BSA to afford porpholactones M5 (Scheme 16). The yields of compounds M46 and M5 are highly dependent on the 2,3-dioxochlorin/BSA ratio and reaction time. When the reaction is carried out in a 1:2 ratio of M44 to BSA, the reaction proceeds very slowly and after 22 h both the ring-contracted azetine and the porpholactone are obtained in low yields while 59% of the unreacted dioxochlorin is recovered (Table 1). However, if a large excess of BSA is used (8-fold) all starting material is consumed within 5 h. The experimental results show that the isolated yield of the ring-contracted product is consistently low, suggesting that this species is an intermediate to the porpholactone M5. In fact, addition of 4 equivalents of BSA to a refluxing chlorobenzene solution of Cu46 generates Cu5 within 30 min; the reaction is complete after 16 h, resulting in 59% yield of Cu5.
Scheme 16.
Synthesis of porphyrinoids reported by Zaleski and co-workers [69].
Table 1.
Yields of the oxidation of 2,3-dioxochlorins M44 under different conditions 1.
| M44 | BSA | Reaction Time | M46 | M5 | M44 Recovered |
|---|---|---|---|---|---|
| Cu44 | 2 equiv | 22 h | 8% | 27% | 59% |
| Cu44 | 4 equiv | 8 h | 7% | 70% | 11% |
| Cu44 | 4 equiv | 18 h | traces | 75% | — |
| Cu44 | 8 equiv | 5 h | 6% | 82% | traces |
| Ni44 | 4 equiv | 14 h | 18% | 14% | 65% |
| Ni44 | 6 equiv | 9 h | 19% | 35% | 20% |
1 Data from ref. [69].
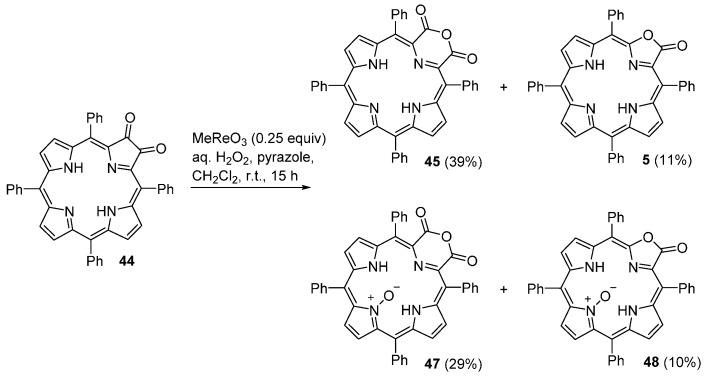
The methyltrioxorhenium (MTO)-catalyzed H2O2 oxidation of the 2,3-dioxochlorin 44, in the presence of pyrazole, leads to the formation of four pyrrole-modified porphyrin derivatives (Scheme 17) [55,70]. During the reaction course it is observed the initial formation of compounds 45 and 5 while the corresponding N-oxides 47 and 48 are formed later in the reaction, and on the expense of the products formed initially [55]. The yield of each product depends on the reaction conditions, namely catalyst loading and reaction time.
Scheme 17.
Synthesis of porphyrinoids and the corresponding N-oxides [55].
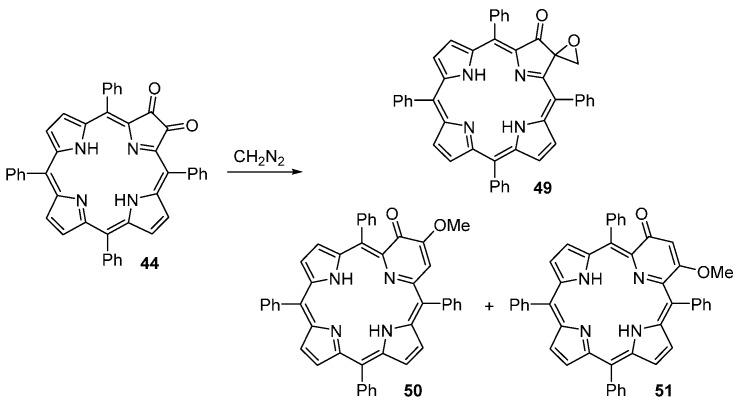
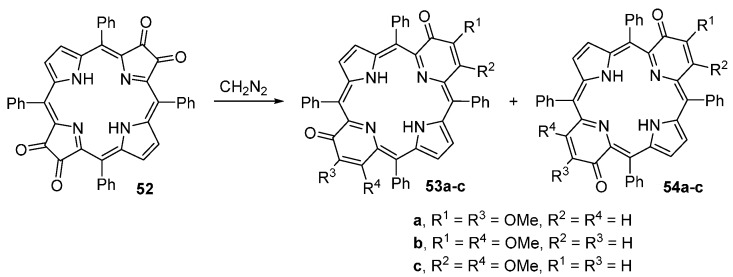
Pandey and co-workers reported a versatile approach to mono- and di(2-oxopyri)porphyrins (Scheme 18 and Scheme 19) [71]. The new compounds are obtained from the reaction of dioxo- and tetraoxo-TPP derivatives with a large excess of diazomethane. The reaction of dioxochlorin 44 with diazomethane affords three products that can be separated by chromatography on silica gel: the 2-oxo-3-epoxymethylenechlorin 49 (7% yield), 3-methoxy-2-oxopyriporphyrin 50 (12% yield) and 4-methoxy-2-oxopyriporphyrin 51 (78% yield). The tetraoxobacteriochlorin 52 reacts immediately with diazomethane to give a mixture of three orange-brown bands that were identified as a mixture of two isomeric di(oxopyri)porphyrins: 53a/54a (18% yield), 53b/54b (31% yield), and 53c/54c (47% yield) (Scheme 19).
Scheme 18.
Synthesis of 2-oxopyriporphyrins [71].
Scheme 19.
Synthesis of di(2-oxopyri)porphyrins [71].
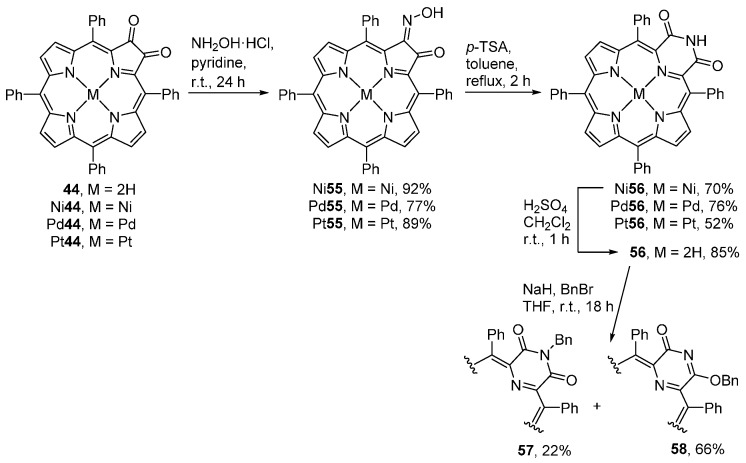
The reaction of 2,3-dioxoporphyrin metal complexes M44 (obtained by metallation of 44) with hydroxylamine hydrochloride affords the corresponding monooximes M55 in good yields (Scheme 20) [72]. When treated with p-toluenesulfonic acid (p-TSA) under forcing conditions, oximes M55 undergo a Beckmann rearrangement to produce the corresponding pyrazinoporphyrin imides M56 in moderate to good yields. Demetallation of pyrazinoporphyrin Ni56 affords the free-base pyrazinoporphyrin imide 56. The reaction of 56 with benzyl bromide in the presence of sodium hydride under nitrogen, at ambient conditions, leads to a mixture of the N-benzyl (22%) and O-benzyl (66%) derivatives 57 and 58, respectively.
Scheme 20.
Synthesis of oximes, Beckmann rearrangement to pyrazinoporphyrin imides and benzylation [72].
Treatment of the free-base oxime 55 (M = 2H) with p-TSA in toluene at reflux leads to the formation of quinoline-annulated porphyrins [73,74].
2.5. From 2-Diazo-3-Oxochlorins
2-Diazo-3-oxochlorins (that can be prepared in good yields from 2-aminoporphyrins [75] or 2,3-dioxochlorins [76]) undergo photodecomposition, with extrusion of N2, to yield a mixture of porphyrin derivatives. The product distribution strongly depends upon the central metal ion and the presence or absence of nucleophilic substrates [77,78,79,80].
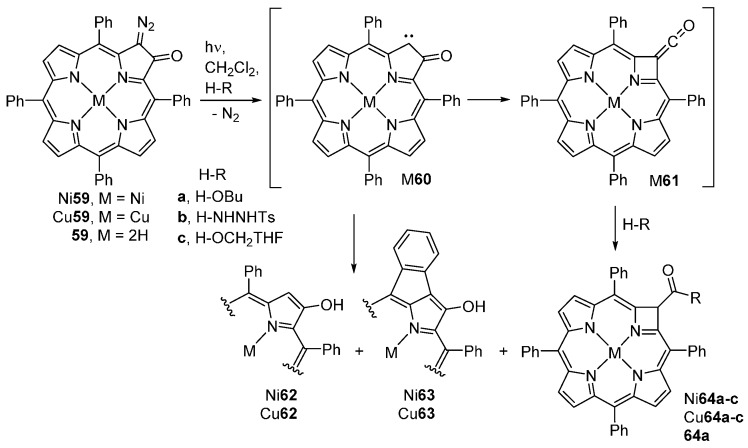
The photolysis of metallated 2-diazo-3-oxochlorins M59 in the absence of nucleophiles affords a mixture of 2-hydroxyporphyrins (M62) and exocyclic ring-containing hydroxyporphyrins (M63) (Scheme 21). In the presence of nucleophiles, the ring-contracted azeteoporphyrins M64 are also obtained. For instance, photolysis of Ni59 in the presence of butan-1-ol, tosylhydrazide, or tetrahydrofurfuryl alcohol yields the Wolff rearranged azeteoporphyrins Ni64a–c (11%–28%), the 2-hydroxyporphyrins Ni62a–c (6%–35%), and the intramolecular exocyclic ring-containing hydroxyporphyrinoids Ni63a–c (12%–76%) [79]. The photolysis of the free-base 2-diazo-3-oxochlorin 59 in the presence of BuOH affords azeteoporphyrin 64a (34%) and a dimeric porphyrin derivative (10%). The formation of ring-contracted azeteoporphyrins M64 involves the Wolff rearrangement of ketocarbene M60 to the ketene M61 that is subsequently trapped with the nucleophiles [81].
Scheme 21.
Products generated by photolysis of 2-diazo-3-oxo-5,10,15,20-tetraphenylchlorins [79].
2.6. From Octaethyl-2-oxochlorins
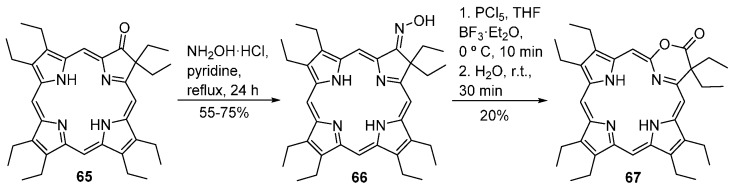
The synthesis of the first 1,3-oxazinochlorin (a pyrrole-modified porphyrin with an 1,3-oxazine ring) was reported recently [82]. The synthetic route involved the conversion of the oxo-chlorin 65, available from octaethylporphyrin, into the corresponding oxime 66 followed by treatment with PCl5 and BF3·Et2O and dropwise addition of H2O under controlled temperature (<25 °C) (Scheme 22). The resulting 1,3-oxazinochlorin 67 was obtained in 20% yield together with some ketone 65 (~20%).
Scheme 22.
Route to 1,3-oxazinochlorins [82].
2.7. From 2,3,12,13-Tetrabromoporphyrins
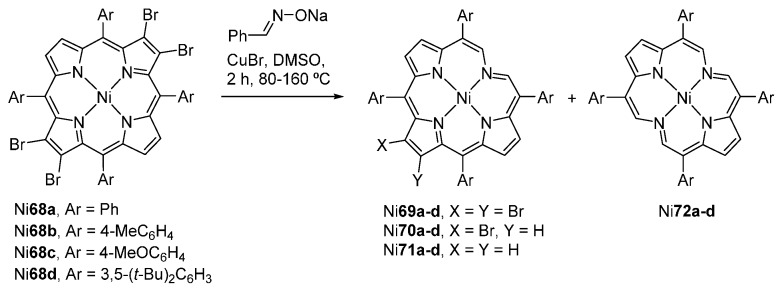
Nickel(II) 2,3,12,13-tetrabromo-5,10,15,20-tetraarylporphyrins Ni68a–d react with the anion of E-benzaldoxime, in the presence of CuBr, to provide the corresponding chlorophins Ni69–Ni71 and bacteriophins Ni72, resulting from the degradation of one or two pyrrolic units, respectively, and mono- or didebromination (Scheme 23) [42,83]. The yield of each product is highly dependent on the time and temperature of the reaction: the best yield of Ni72a (26%) is achieved after 2 h at a temperature fluctuating regularly between 80 °C and 160 °C. These chlorophins and bacteriophins display UV-vis spectra similar to those of metallated chlorin and bacteriochlorin systems but with more intense and bathochromically shifted Q-bands.
Scheme 23.
Route to chlorophins and bacteriophins [83].
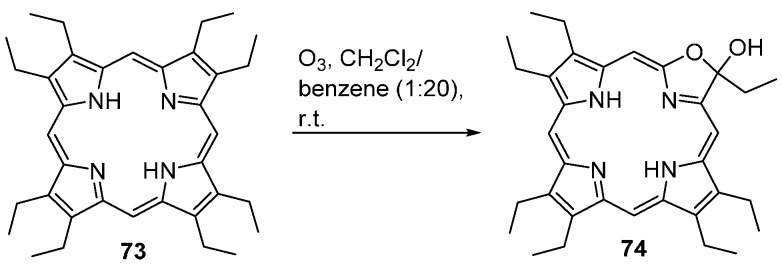
2.8. From β-Unsubstituted Porphyrins
Octaethylporphyrin (73) reacts with ozone at room temperature to afford the oxazolochlorin 74 (Scheme 24) [84]. This is probably the first reported example of a direct conversion of a porphyrin into a pyrrole-modified porphyrin.
Scheme 24.
Route to oxazolochlorins [84].
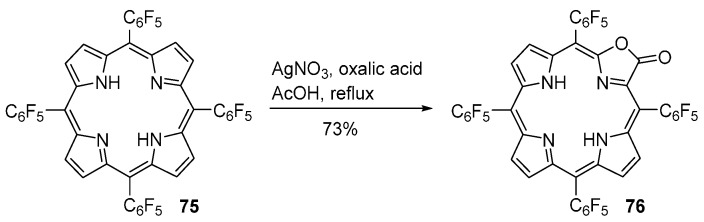
Gouterman and co-workers discovered, by serendipity, the direct conversion of free-base meso-tetraarylporphyrins into porpholactones using mild reagents [85]. While attempting to synthesize the silver complex of meso-tetrakis(pentafluorophenyl)porphyrin (AgF20TPP) by treatment of the corresponding free-base H2F20TPP 75 with AgNO3 in glacial acetic acid at reflux, they obtained (in low yield) the corresponding porpholactone 76 (Scheme 25). These authors found that addition of oxalic acid to the reaction mixture allows the synthesis of porpholactone 76 in reproducible yields (ca. 15%) [85]. Later, the yield of this reaction was improved to 73% [86]. Porpholactone 79 can be converted into the corresponding Ni, Zn, Pd and Pt complexes by standard procedures [86].
Scheme 25.
Route to meso-tetrakis(pentafluorophenyl)porpholactone [85,86].
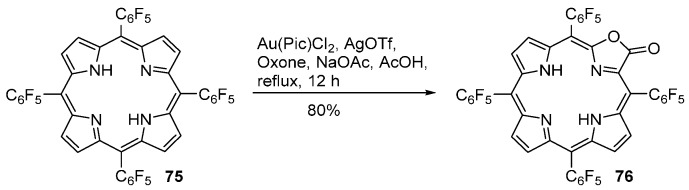
Zhang and co-workers [87] found that heating a solution of H2F20TPP 75 and HAuCl4 (2 equiv.) in acetic acid at reflux affords a mixture of [Au(F20TPP)]+ (20% yield), 2-chloro-H2F20TPP (25% yield) and the porpholactone 76 (6% yield). However, under similar conditions, TPP affords only the corresponding gold complex [AuTPP]+ in 85% yield. Using 2-picolinic acid (Pic) as ligand, the porpholactone 76 is obtained in 63% yield (yield calculated based on the conversion (52%) of the starting porphyrin). A further improvement of the porpholactone yield is achieved by adding an oxidant to the reaction mixture. The highest yield of porpholactone 76 (80%) is obtained when two equivalents of Oxone® are used, although the conversion of H2F20TPP was low (64%) (Scheme 26). Under these conditions, the complex [AuF20TPP]+ is formed in very low yield (<2%). In the absence of [Au(Pic)Cl2], the reactivity of Oxone® toward H2F20TPP is very low (<5%), confirming the catalytic effect of Au(III). Other fluorinated meso-tetraarylporphyrins can be converted into porpholactones by this gold-catalyzed approach. However, the electronic effect of the substituents of the porphyrins is very pronounced since the yield of the gold porphyrin complex increases and consequently the yield of the porpholactone reduces drastically in the order: meso-tetrakis(tetrafluorophenyl)-, meso-tetrakis(trifluorophenyl)-, meso-tetrakis(difluorophenyl)-, and meso-tetrakis(p-fluorophenyl)porphyrin. The electronic effect of the substituents may be minimized if Ag+ is used as a template metal ion. Starting from AgTPP, Ag(p-ClTPP) and Ag(p-FTPP) the corresponding free-base porpholactones are obtained in 23%, 17% and 10% isolated yields [87].
Scheme 26.
Gold-catalyzed synthesis of meso-tetrakis(pentafluorophenyl)porpholactone [87].
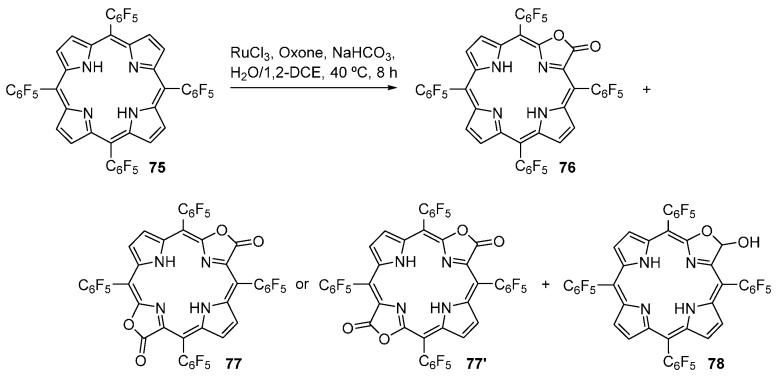
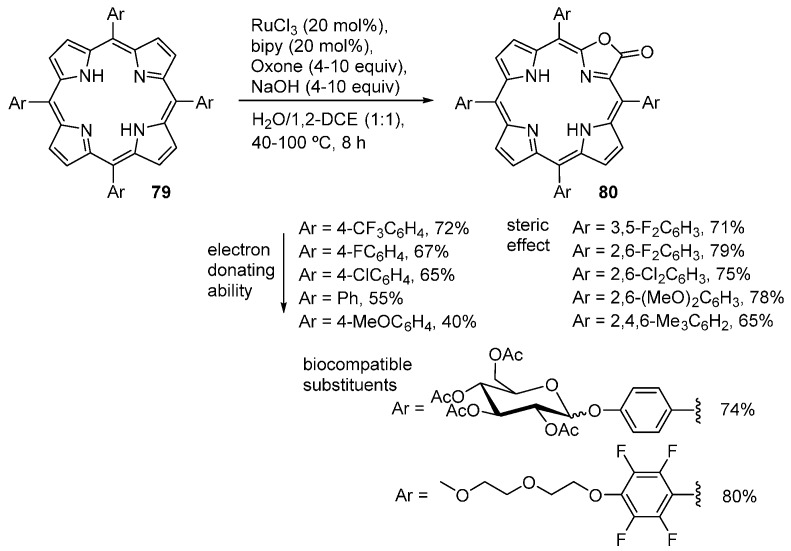
Zhang and co-workers also investigated the efficacy of RuCl3 as catalyst in the direct conversion of meso-tetraarylporphyrins to the corresponding porpholactones [88,89]. Using H2F20TPP as substrate, RuCl3 as catalyst and Oxone®/NaHCO3 as oxidant, these authors obtained a mixture of porpholactone 79 (32% yield), porphodilactones 77/77’ (4% yield) and porpholactol 78 (2% yield) (Scheme 27). This reaction system was optimized in order to improve the reactivity and chemoselectivity. The best yield of porpholactone 76 (85%) was obtained using RuCl3 (20 mol%), bipyridine (20 mol %, as a ligand), Oxone® (5 equiv.) and sodium hydroxide (5 equiv.) and a reaction temperature of 60 °C.
Scheme 27.
Ruthenium-catalyzed oxidation of meso-tetrakis(pentafluorophenyl)porphyrin [88,89].
This methodology can also be applied successfully to meso-tetraarylporphyrins bearing substituents with different electronic and steric effects and biocompatible substituents, as shown in Scheme 28. The corresponding porpholactones 80 are obtained in 40%–80% isolated yields [88]. Metalloporphyrins can also be converted into metalloporpholactones by this protocol. Using MF20TPP (M = Ni, Cu, Zn, Pd) as substrates, the corresponding metalloporpholactones are obtained in high yields (78%–85%). However, oxidation of PtF20TPP gives a mixture of Pt(porpholactone) (30% yield) and the free-base porpholactone (46% yield) while no gold(III) porpholactone is detected from the oxidation of [AuF20TPP]Cl [88].
Scheme 28.
Ruthenium-catalyzed oxidation of meso-tetraarylporphyrins [88].
2.9. Modification of Other Porphyrinoids
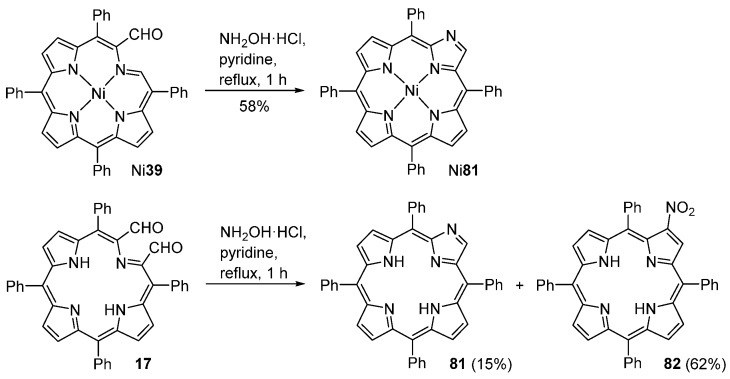
The reaction of chlorophin-2-carbaldehyde Ni39 with an excess of hydroxylamine hydrochloride in refluxing pyridine, for 1 h, generates the imidazoloporphyrin Ni81 in 58% yield (Scheme 29) [90]. The free-base imidazoloporphyrin 81 was obtained in 15% yield from the reaction of secochlorin-2,3-dicarbaldehyde 17 with hydroxylamine hydrochloride in refluxing pyridine. The main product of this reaction is, however, 2-NO2TPP (82, 62%).
Scheme 29.
Conversion of chlorophins and secochlorins into imidazoloporphyrins [90].
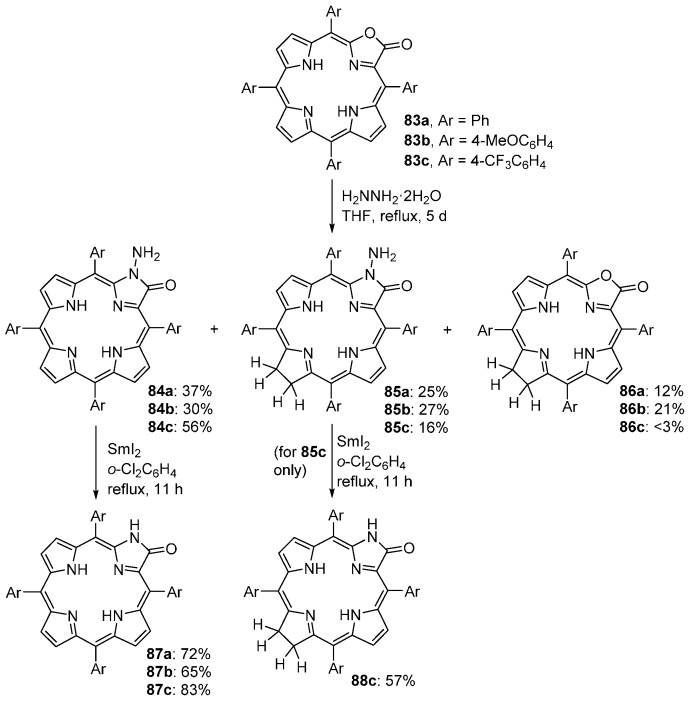
meso-Tetraarylporpholactones 83 react with hydrazine hydrate, in THF solution, to form three products: the N-aminoporpholactams 84 (main products), the N-aminochlorolactams 85, and the chlorolactones 86 (minor products) (Scheme 30) [91,92]. The reaction requires a large excess of hydrazine hydrate and heating at reflux for 3–5 days. The reductive cleavage of the N-N bond in N-aminoporpholactams 84 or N-aminochlorolactams 85, using SmI2 in refluxing o-dichlorobenzene, affords the parent porpholactams 87 or 88, respectively, in good yields.
Scheme 30.
Conversion of porpholactones into N-aminoporpholactams and porpholactams [92].
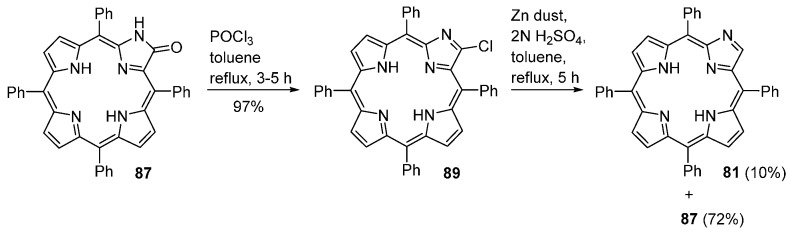
Porpholactams can be further modified to imidazoloporphyrins, as shown in Scheme 31. In fact, treatment of porpholactam 87 with phosphoryl chloride (POCl3) in refluxing toluene gives access to the 3-chloroimidazoloporphyrin 89 in 97% yield. Subsequent reaction of a toluene solution of 89 with zinc dust and 2 N aq. H2SO4, at reflux for 5 h, affords a mixture of imidazoloporphyrin 81 (10% yield) and the starting porpholactam 87 (72%) [92].
Scheme 31.
Conversion of porpholactams into imidazoloporphyrins [92].
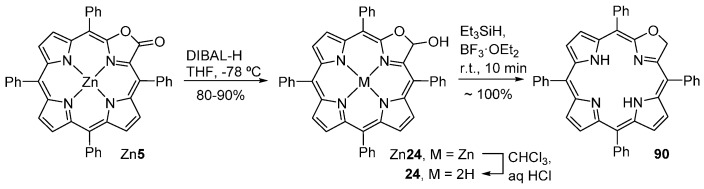
Reduction of the porpholactone Zn5 with DIBAL-H affords the porpholactol Zn24 that, by demetallation, provides the free-base porpholactol 24 in almost quantitative yield (Scheme 32) [54,93,94]. The acid-catalyzed (BF3·OEt2 or Amberlyst 15) deoxygenation of the porpholactol 24 affords the 2-oxachlorin 90 in near-quantitative yield. Over time, this compound undergoes (photo)oxidation back to porpholactol 24. Thus, it must be shielded from exposure to light or oxidizing conditions. Attempts at one-step reductions from Zn5 to Zn90 using LiAlH4 or DIBAL-H under more forcing conditions failed, leading to the destruction of the porphyrinic macrocycle [54].
Scheme 32.
Stepwise reduction of a porpholactone to a porpholactol and a 2-oxachlorin [94].
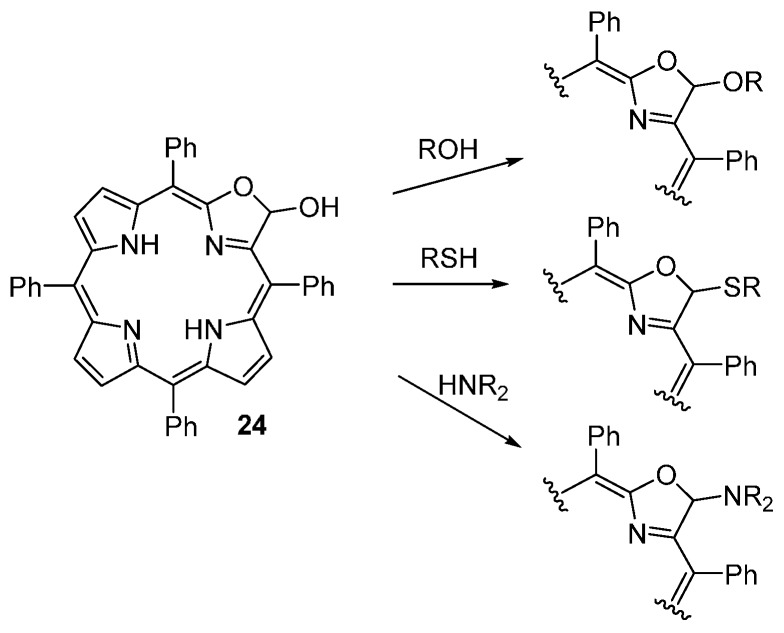
The porpholactol 24 (an hemiacetal) reacts with a range of O-, N-, and S- nucleophiles, under mild acid-catalyzed conditions, providing access to a number of stable chlorin-like acetals, thioacetals, and aminals (Scheme 33) [54].
Scheme 33.
Derivatization of porpholactol 24 with O-, N-, and S- nucleophiles [54].
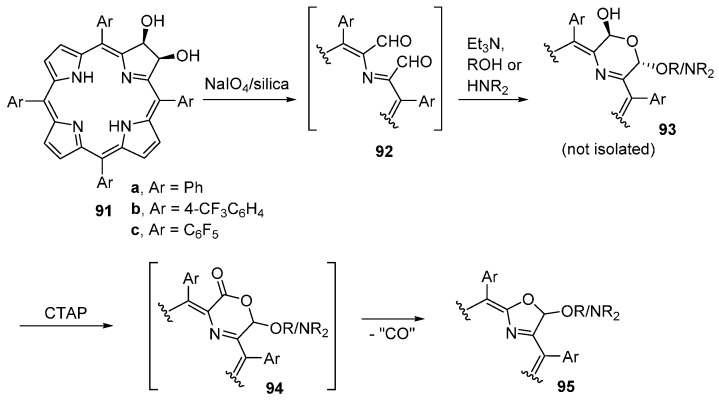
Acetals and aminals 95 can be prepared in a two-step, one-pot reaction directly from 2,3-dihydroxychlorins 91 (Scheme 34) [95]. The first step corresponds to the oxidation of 2,3-dihydroxychlorins 91 to secochlorin-2,3-dicarbaldehydes 92 (see Scheme 7) in the presence of a nucleophile (alcohol or secondary amine). The resulting crude mixture of morpholinochlorins 93 is then oxidized with CTAP. Depending on the nucleophile used in the first step, oxazolochlorin acetals or aminals are formed in good to acceptable isolated yields. The mechanism of the conversion of morpholinochlorins 93 into the oxazolochlorin derivatives 95 probably involves the formation of morpholinones 94 and extrusion of CO. Comparing this synthetic route to acetals and aminals with the previous one, the number of steps was reduced from 5 to 2 and the overall yields more than doubled: 73%–91% for the acetals and 33%–49% for the aminals.
Scheme 34.
One-pot synthesis of oxazolochlorin acetals and aminals [95].
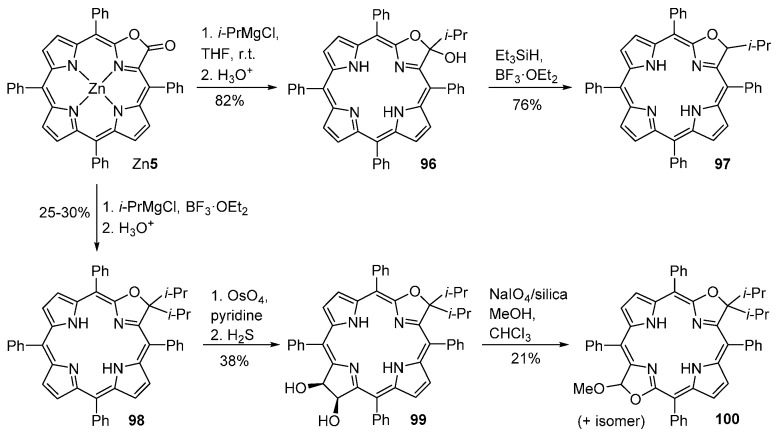
Porpholactones react with alkyl-Grignard reagents to afford 3-alkyl- or 3,3-dialkyloxazolochlorins [96,97,98]. Addition of i-PrMgCl (15 equivalents) to the zinc complex of porpholactone Zn5, followed by an acid workup (that also removes the Zn(II)), affords the hemiketal 96 in 82% yield (Scheme 35). Reaction of 96 with an excess of Et3SiH, in the presence of BF3·OEt2, leads to the formation of the 3-isopropyloxazolochlorin 97 in 76% yield. Addition of an excess of i-PrMgCl to porpholactone Zn5 in the presence of BF3·OEt2 gives the diisopropyloxazolochlorin 98 in ca. 30% yield. Oxazolochlorins 96–98 can be further converted into bacteriochlorin-type compounds, such as 99 and 100 [96,97,98]. Oxazolochlorins 96–98 and the corresponding hydroxylated oxazolobacteriochlorins possess typical chlorin and bacteriochlorin-like optical spectra with absorption bands between 650–750 nm.
Scheme 35.
Addition of alkyl-Grignard reagents to porpholactones and further transformation into bisoxazolobacteriochlorins [96,97,98].
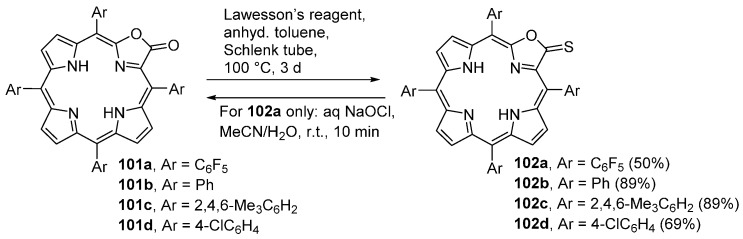
Reaction of porpholactones 101a–d with an excess of Lawesson’s reagent leads to the formation of the corresponding porphothionolactones 102a–d in good to excellent yields (Scheme 36) [99]. The porphothionolactone 102a can be converted to the corresponding lactone 101a rapidly and quantitatively by addition of aqueous NaOCl (bleach). Since porpholactones are moderately fluorescent and the porphothionolactones are not, any reaction that induces the thionolactone-to-lactone conversion is accompanied by a strong fluorescence emission intensity enhancement. This process is the basis for the use of thionolactone 102a as a switch-on chemodosimeter for hypochlorite (see Section 3.4. Optical Sensors).
Scheme 36.
Conversion of porpholactones into porphothionolactones [99].
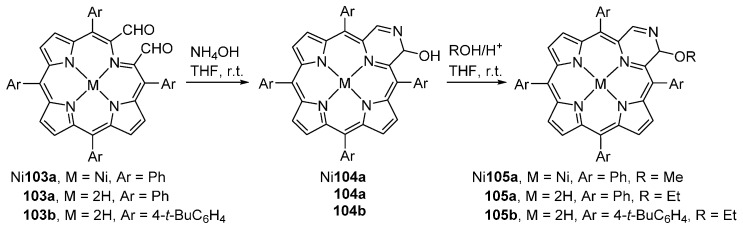
Reaction of secochlorin Ni103a with aqueous concentrated ammonia in THF, at room temperature, for 20 minutes, generates the pyrazinoporphyrin hemiacetal Ni104a in ca. 90% yield. Treatment of crude Ni104a with MeOH and catalytic quantities of THF generates the methoxy pyrazinoporphyrin Ni105a (Scheme 37) [100]. The free-base pyrazinoporphyrins 105a,b can be prepared in a similar way. Treatment of the secochlorins 103a,b under aerobic conditions with an excess of aqueous concentrated ammonia in pyridine at 40–50 °C, leads to the rapid formation of the pyrazinoporphyrin hemiacetals 104a,b. While the Ni(II) complex Ni105a is stable even under acidic conditions, the free-base analogues are very sensitive to decomposition, particularly under acidic or oxidative conditions. The extinction coefficients of the pyrazinoporphyrins are significantly smaller than those of TPP.
Scheme 37.
Route to pyrazinoporphyrins [100].
3. Applications
Pyrrole-modified porphyrin derivatives have recently gained an increasing interest due to their potential application in photodynamic therapy (PDT), NIR-absorbing dyes, multimodal imaging contrast agents, catalysis, high pH sensors, optical sensors for cyanide, hypochlorite, and oxygen.
3.1. Photodynamic Therapy
Photodynamic therapy (PDT) is a promising strategy in cancer treatment. It uses a photosensitizer that, upon cellular internalization and light irradiation, generates reactive oxygen species (ROS) (mainly singlet oxygen) that kill the cancer cells leading to the eradication of the tumor. Porphyrin derivatives, namely chlorins and bacteriochlorins, due to their photophysical properties, such as strong absorption bands in the range of 650–800 nm, and appropriate triplet state to generate ROS, are particularly useful as photosensitizers. Pyrrole-modified porphyrins that display absorption spectra typical of chlorins or bacteriochlorins are also interesting compounds to be used as photosensitizers in PDT. Recent studies confirm the superior properties of such compounds.
In 2005, McCarthy and co-workers described the encapsulation of meso‑tetraphenylporpholactol (24), a chlorin-type compound, into a poly(lactic-co-glycolic acid) (PLGA) matrix and the use of the resulting nanoparticles as photosensitizers in the PDT of tumors [94]. These authors found that the PLGA/porpholactol nanoparticles are unable to fluoresce or induce phototoxicity. However, upon cell internalization, the porpholactol is released from the nanoparticle, regains its ability to fluoresce and produce singlet oxygen, and becomes highly phototoxic. Irradiation with visible light results in cell-specific killing of several cancer cell lines. Importantly, in vivo experiments with this activatable PDT-nanoagent resulted in the complete eradication of cancers in mouse models.
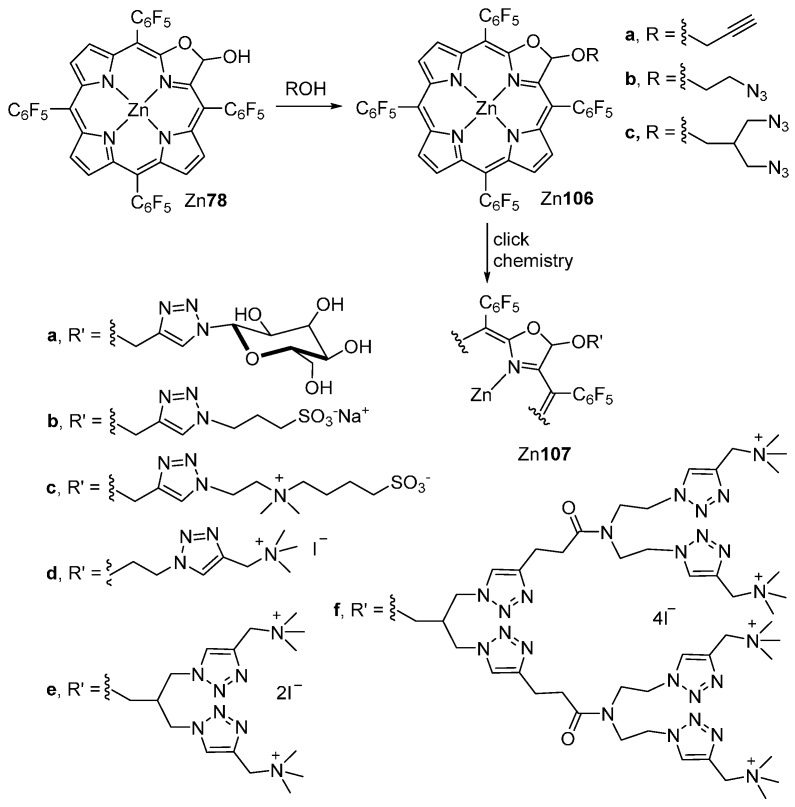
Recently, Zhang and co-workers [101] reported the synthesis and evaluation of the potentialities of six porpholactol-type compounds (oxazolochlorin acetals) as PDT photosensitizers. The new compounds were prepared by chemical modification of porpholactol Zn78 (Scheme 38) and bear β-hydrophilic substituents terminated by glucosyl (neutral, Zn107a), sulfonic (anionic, Zn107b), zwitterionic (Zn107c), or ammonium (cationic, Zn107d–f) groups. It was shown that the terminal ionic groups influence significantly the cellular uptake of these conjugates that is higher for the compounds with positive charge. Importantly, their photocytotoxicity against cancer cells showed IC50 values down to the sub-micromolar range being the cationic compounds the most active ones. As revealed by cell imaging experiments, these compounds exert their photodynamic activity through apoptosis.
Scheme 38.
Porpholactol-type compounds studied as photosensitizers in PDT [101].
Porpholactones have also been studied as PDT photosensitizers. Zhang and co-workers compared the photophysical and biological properties of meso-tetrakis(pentafluorophenyl)porphyrin (75), the corresponding porpholactone 76 and their derivatives bearing thioglucosyl units [102]. This study demonstrated that the β-lactonization of the porphyrin lowers its lipophilicity and increases its binding affinity to low density lipoproteins facilitating its cellular uptake and selective localization within lysosomes. Lactonization also leads to enhanced singlet oxygen quantum yields, thus increasing the intracellular ROS levels.
The same research group also reported the photosensitizing properties of the two isomeric cis- (77) and trans- (77’) porphodilactones (Scheme 27) and the corresponding Zn(II) complexes [89]. The electronic absorption spectra of the two isomers are similar to those of bacteriochlorins, with strong absorption bands in NIR region (>650 nm). However, the authors found that the relative orientation of the two β-lactone moieties has a significant influence on the electronic structures and photophysical properties of the porphodilactones. For example, the Qy band of trans-porphodilactone is red-shifted by 19 nm relative to that of the cis-isomer, and there is a 2-fold increase in the absorption intensity [89]. The orientation of the two lactone moieties also affects the singlet oxygen production of the two isomeric porphodilactones. The values obtained for the singlet oxygen quantum yields (ΦΔ) were 0.53 for the free-base trans-porphodilactone 77’, 0.66 for Zn77’, and 0.87 for Zn77. Surprisingly, the free-base cis-porphodilactone 77 shows no singlet oxygen production at all after irradiation by a 635 nm laser beam. Both porphodilactones, encapsulated into PLGA nanoparticles, showed no dark toxicity after incubation for 24 h in HeLa cells. However, after irradiation with a 671 nm laser for 60 s, porphodilactones 77’ and Zn77’ exhibited good phototoxicity with a IC50 value of 51 and 44 μg·mL−1, respectively. The IC50 value of Zn77 was significantly larger (94 μg·mL−1), despite having a larger ΦΔ value, while the free-base cis-porphodilactone 77 showed nearly no photocytoxicity toward HeLa cells, which was consistent with the zero singlet oxygen production [89].
3.2. Multimodal Imaging Contrast Agents
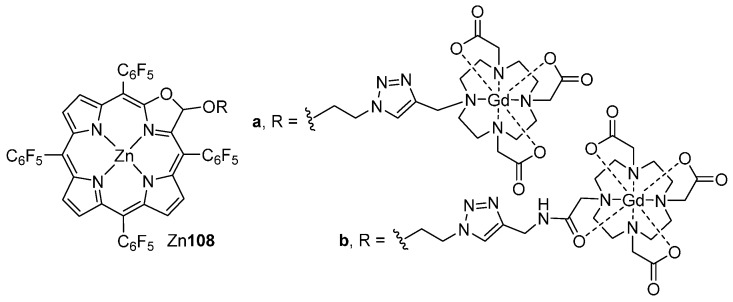
Nowadays, there is a need to develop theranostic compounds for simultaneous cancer imaging, diagnosis and treatment interventions. In this context, and following the procedure indicated in Scheme 38, the group of Zhang prepared two oxazolochlorin acetals bearing gadolinium(III) DOTA-type complexes (Zn108a,b, Figure 2) [103]. Both compounds can achieve cellular uptake, show no dark cytotoxicity and good photocytotoxicity on HeLa cells. Fluorescence and magnetic resonance imaging experiments showed that both compounds are suitable as bimodal imaging contrast agents. In addition, the photocytotoxicity results indicate that these compounds can also be exploited as photosensitizers for PDT.
Figure 2.
Porpholactol-type compounds studied as multimodal imaging contrast agents [103].
3.3. Catalysis
The first report about the use of metalloporpholactones in catalysis dates back to 2005, when Cetin and Ziegler [104] demonstrated the catalytic activity of the manganese(III) complex of meso-tetraphenylporpholactone, Mn(TPPL)Cl (Mn5-Cl), in olefin epoxidation reactions. Comparing with the manganese(III) complex of meso-tetraphenylporphyrin, Mn(TPP)Cl, the Mn(TPPL)Cl is a slightly better catalyst for most alkene substrates. However, for the electron-poor substrate hex-1-ene, the turnover number is appreciably larger than the one observed for the manganese porphyrin.
Iron(III) and manganese(III) complexes of meso-tetraphenylporpholactone, Fe(TPPL)Cl and Mn(TPPL)CH3CO2, are efficient catalysts for the oxidation of sulfides with hydrogen peroxide [105]. Both aliphatic and aromatic sulfides are effectively oxidized to the corresponding sulfoxides and sulfones in yields of 80%–100%. The results show that the iron and manganese porpholactones catalyze the oxidation of sulfides with similar efficiency and are slightly better catalysts than the TPP analogues for most substrates.
The iron(III) complex of meso-tetrakis(pentafluorophenyl)porpholactone, Fe(F20TPPL)Cl (Fe76-Cl), is an effective catalyst for nitrogen atom transfer reactions such as aziridination of alkenes and amidation of alkanes using organic azides [106]. In contrast, the manganese(III) complex of the same porpholactone shows a low catalytic activity.
The palladium(II) complex of meso-tetraphenylporpholactone (Pd5) and other Pd(II)-porphyrin complexes were studied as catalysts for the light-induced aerobic oxidation of dibenzylamine to the corresponding imine [107]. The results showed that while PdF20TPP could furnish 100% substrate conversion (99% yield) within 1.5 h, the substrate conversion using Pd5 was only 75% (74% yield) for a similar reaction time. Still, this substrate conversion was higher than the observed for PdTPP (38%, 37% yield), indicating that the lactonization of the porphyrin macrocycle was beneficial for the photocatalysis.
3.4. Optical Sensors
Porphyrins and related macrocycles play a preeminent role in sensing applications involving chromophores [108]. Pyrrole-modified porphyrins are also becoming more frequent as chemo(sensors). For instance, the Pt(II) complexes of the meso-tetrakis(pentafluorophenyl)porphyrin (Pt75) and of the corresponding porpholactone (Pt76) have been extensively used as the O2-sensitive components of pressure sensitive paints [109,110,111,112,113]. The porphothionolactone 102a (Scheme 36) is another example. This compound is an effective fluorescent switch-on chemodosimeter for hypochlorite (OCl−) [99]. In fact, this porphothionolactone is rapidly and quantitatively converted into the corresponding porpholactone 101a by the addition of an aqueous solution of NaOCl (bleach). Since the thionolactone possesses only a very dim fluorescence emission but the lactone is brightly fluorescing, this reaction switches the fluorescence on (readily observed with the naked eye) within 10 min at ambient conditions. The conversion of the thionolactone to the lactone is highly selective for the oxidant hypochlorite and that is the basis for the use of this thionolactone as a switch-on chemodosimeter for this anion. However, it must be taken into consideration that this reaction is pH-dependent and acidic to neutral conditions (pH between 1 and 7.4) are required for the conversion of 102a by OCl− [99].
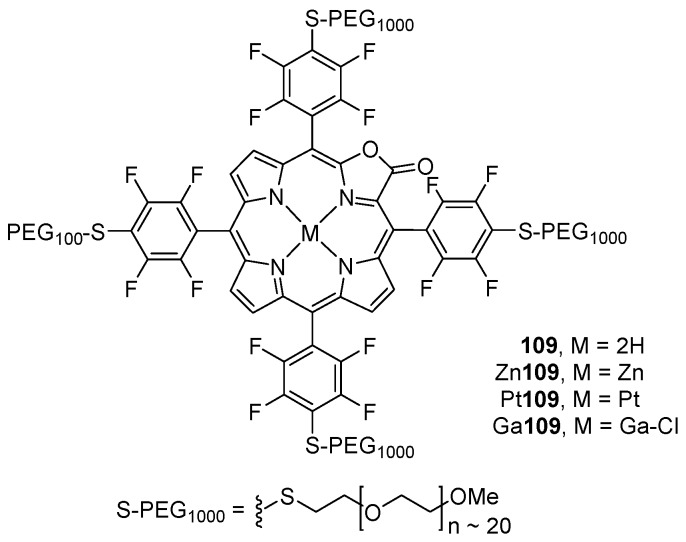
Cyanide is one of the most toxic and harmful anions to human health and environment but it is still widely used in mining, metallurgy, photographic processing, synthesis of nylon and other synthetic fibers, etc. Thus, it is very important to develop simple, selective, and sensitive methods to the detection of CN−, especially in water [114]. One interesting example of the potential applications of the pyrrole-modified porphyrins is their use as optical cyanide chemosensors. Recently, the group of Brückner demonstrated that the water-soluble PEGylated porpholactone 109 and its zinc(II), platinum(II), and gallium(III) complexes (Figure 3) behave as optical cyanide chemosensors in aqueous solutions, albeit these sensors possess only modest sensitivities [115]. It was shown that the optical response of the Zn109 complex to cyanide is totally different from that of the free-base, platinum, and gallium complexes. While for the Zn109 it can be attributed to axial ligation of the cyanide to the central metal, the sensing mechanism for 109, Pt109 and Ga109 relies on a nucleophilic addition of the cyanide to the lactone moiety. Incorporation of the gallium porpholactone complex (Ga109) in a Nafion® membrane resulted in a material that shows a reversible response to CN− and remains stable and transparent even after days in aqueous solutions [115].
Figure 3.
Water-soluble PEGylated porpholactones used as optical cyanide sensors [115].
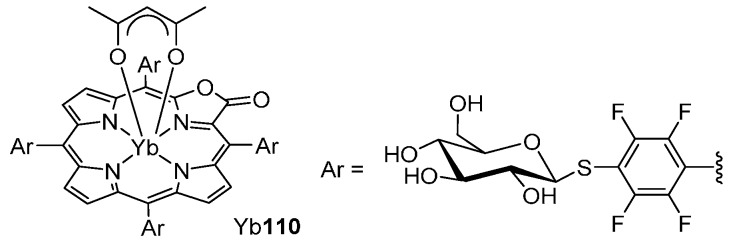
A recent study demonstrated that β-lactonization of porphyrin ligands enhances the sensitization efficiency of the NIR luminescence of the ytterbium(III) complexes (Figure 4) [116]. In addition, it was found that the NIR emission of the water-soluble Yb(porpholactone)-glucose conjugate Yb107 is specifically switched on in the presence of glucose oxidase and is switched off by addition of glucose. This experiment shows that ytterbium complex Yb110 may find application as a switchable probe emitting in the NIR region in which biological tissues and fluids are relatively transparent (900–1100 nm).
Figure 4.
Water-soluble NIR luminescent Yb(porpholactone) complex [116].
The ability of meso-tetrakis(pentafluorophenyl)porpholactone (76) and its Pt(II) complex to function as optical high pH sensors was reported by the group of Brückner [53]. This group found that these compounds behave as chemosensors for OH− and MeO−. When submitted to strong alkaline or high methoxide conditions their UV-vis spectra undergo dramatic and reversible red-shifts. The dynamic range for the Pt76 in solution is from pH 11.5 to 13.2. This complex can also be used to coat optical fibers, allowing the construction of optical fiber-based pH sensors.
This study was extended to a range of metal complexes of other meso-tetraarylporpholactones, allowing to investigate the influence of the metal (M = Zn(II), Ni(II), Cu(II), Pd(II), Ag(II), Pt(II)), the aryl group (Ar = Ph, C6F5), and the presence of a β-NO2 group, on the pH sensing range [117]. The results showed that the optical response for the pentafluorophenyl-substituted derivatives is stronger than that of the nitrated phenyl-substituted analogues. However, β-nitration and pentafluorophenyl-substitution have additive effects. Since the metal has a minor influence on the optical response, the cheaper and easier to prepare zinc, copper and silver complexes of porpholactones are excellent alternatives to the platinum complexes [117].
PEGylated and fully water-soluble Pt(II)- and Ga(III)porpholactones behave as high pH sensors in purely aqueous solutions (pH between 10 and 12.5 and 8 and 11, respectively) [118]. Incorporation of these compounds into a Nafion® optode membrane resulted in sensors suitable for a moderately rapid (response time in minutes) sensing of high concentrations of hydroxides (pH 11 and above).
4. Conclusions
As discussed above, the pyrrole-modified porphyrin chemistry is evolving quickly. Several methods to access these compounds, and new reactions for their conversion into other porphyrinoids, were already reported. Also, various (potential) applications have been suggested for these types of compounds and, in many cases, these porphyrinoids show a better performance than their porphyrin analogues. However, considering that these studies have been developed by a very restricted number of research groups, for a faster development of the pyrrole-modified porphyrins field (chemistry and applications) it is crucial that other research groups may become interested in this topic. Thus, we expect that this review contributes to motivate other researchers to embrace this subject.
Acknowledgments
Thanks are due to Fundação para a Ciência e a Tecnologia (FCT/MEC), European Union, QREN, FEDER and COMPETE for funding the project PTDC/QEQ-QOR/1273/2012 and the QOPNA research unit (FCT UID/QUI/00062/2013; FCOMP-01-0124-FEDER-037296). Letícia D. Costa is grateful to FCT for a research grant.
Abbreviations
- BSA
benzeneselenic anhydride
- CTAP
cetyltrimethylammonium permanganate
- DDQ
2,3-dichloro-5,6-dicyano-1,4-benzoquinone
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- PDT
photodynamic therapy
- Pic
2-picolinic acid
- PLGA
poly(lactic-co-glycolic acid)
- ROS
reactive oxygen species
- TPP
meso-tetraphenylporphyrin
- H2F20TPP
meso-tetrakis(pentafluorophenyl)porphyrin
- p-TSA
p-toluenesulfonic acid
Author Contributions
All authors contributed to the writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- 1.Srivatsan A., Missert J.R., Upadhyay S.K., Pandey R.K. Porphyrin-based photosensitizers and the corresponding multifunctional nanoplatforms for cancer-imaging and phototherapy. J. Porphyr. Phthalocyanines. 2015;19:109–134. doi: 10.1142/S1088424615300037. [DOI] [Google Scholar]
- 2.Almeida A., Cunha A., Faustino M.A.F., Tomé A.C., Neves M.G.P.M.S. Porphyrins as antimicrobial photosensitizing agents. In: Hamblin M.R., Jori G., editors. Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications. Royal Society of Chemistry; London, UK: 2011. pp. 83–160. [Google Scholar]
- 3.Costentin C., Robert M., Savéant J.-M. Current issues in molecular catalysis illustrated by iron porphyrins as catalysts of the CO2-to-CO electrochemical conversion. Acc. Chem. Res. 2015;48:2996–3006. doi: 10.1021/acs.accounts.5b00262. [DOI] [PubMed] [Google Scholar]
- 4.Mori H., Tanaka T., Osuka A. Fused porphyrinoids as promising near-infrared absorbing dyes. J. Mater. Chem. C. 2013;1:2500–2519. doi: 10.1039/c3tc00932g. [DOI] [Google Scholar]
- 5.Goslinski T., Piskorz J.J. Fluorinated porphyrinoids and their biomedical applications. Photochem. Photobiol. C. 2011;12:304–321. doi: 10.1016/j.jphotochemrev.2011.09.005. [DOI] [Google Scholar]
- 6.Lash T.D. Benziporphyrins, a unique platform for exploring the aromatic characteristics of porphyrinoid systems. Org. Biomol. Chem. 2015;13:7846–7878. doi: 10.1039/C5OB00892A. [DOI] [PubMed] [Google Scholar]
- 7.Lash T.D. Carbaporphyrins, porphyrin isomers and the legacy of Emanuel Vogel. J. Porphyrins Phthalocyanines. 2012;16:423–433. doi: 10.1142/S1088424612300017. [DOI] [Google Scholar]
- 8.Arnold L., Müllen K. Modifying the porphyrin core-a chemist’s jigsaw. J. Porphyrins Phthalocyanines. 2011;15:757–779. doi: 10.1142/S1088424611003720. [DOI] [Google Scholar]
- 9.Lash T.D. Recent advances on the synthesis and chemistry of carbaporphyrins and related porphyrinoid systems. Eur. J. Org. Chem. 2007:5461–5481. doi: 10.1002/ejoc.200700478. [DOI] [Google Scholar]
- 10.Szyszko B., Latos-Grazynski L. Core chemistry and skeletal rearrangements of porphyrinoids and metalloporphyrinoids. Chem. Soc. Rev. 2015;44:3588–3616. doi: 10.1039/C4CS00398E. [DOI] [PubMed] [Google Scholar]
- 11.Lash T.D. Metal complexes of carbaporphyrinoid systems. Chem. Asian J. 2014;9:682–705. doi: 10.1002/asia.201301594. [DOI] [PubMed] [Google Scholar]
- 12.Pacholska-Dudziak E., Latos-Grazynski L. Aza-deficient porphyrin as a ligand. Coord. Chem. Rev. 2009;253:2036–2048. doi: 10.1016/j.ccr.2009.01.029. [DOI] [Google Scholar]
- 13.Tomé A.C., Neves M.G.P.M.S., Cavaleiro J.A.S. Porphyrins and other pyrrolic macrocycles in cycloaddition reactions. J. Porphyrins Phthalocyanines. 2009;13:408–414. doi: 10.1142/S1088424609000619. [DOI] [Google Scholar]
- 14.Cavaleiro J.A.S., Neves M.G.P.M.S., Tomé A.C. Cycloaddition reactions of porphyrins. Arkivoc. 2003;xiv:107–130. [Google Scholar]
- 15.Costa J.I.T., Tomé A.C., Neves M.G.P.M.S., Cavaleiro J.A.S. 5,10,15,20-Tetrakis(pentafluorophenyl) porphyrin: A versatile platform to novel porphyrinic materials. J. Porphyr. Phthalocyanines. 2011;15:1116–1133. doi: 10.1142/S1088424611004294. [DOI] [Google Scholar]
- 16.Tomé A.C., Lacerda P.S.S., Neves M.G.P.M.S., Cavaleiro J.A.S. meso-Arylporphyrins as dienophiles in Diels–Alder reactions: A novel approach to the synthesis of chlorins, bacteriochlorins and naphthoporphyrins. Chem. Commun. 1997:1199–1200. doi: 10.1039/a702504a. [DOI] [Google Scholar]
- 17.Vicente M.G.H., Cancilla M.T., Lebrilla C.B., Smith K.M. Cruciform porphyrin pentamers. Chem. Commun. 1998:2355–2356. doi: 10.1039/a806563b. [DOI] [Google Scholar]
- 18.Silva A.M.G., Tomé A.C., Neves M.G.P.M.S., Cavaleiro J.A.S. Novel barrelene-fused chlorins by Diels–Alder reactions. Tetrahedron Lett. 2000;41:3065–3068. doi: 10.1016/S0040-4039(00)00336-1. [DOI] [Google Scholar]
- 19.Silva A.M.G., Tomé A.C., Neves M.G.P.M.S., Cavaleiro J.A.S., Kappe C.O. Porphyrins in Diels–Alder reactions. Improvements on the synthesis of barrelene-fused chlorins using microwave irradiation. Tetrahedron Lett. 2005;46:4723–4726. doi: 10.1016/j.tetlet.2005.05.047. [DOI] [Google Scholar]
- 20.Zhao S., Neves M.G.P.M.S., Tomé A.C., Silva A.M.S., Cavaleiro J.A.S., Domingues M.R.M., Correia A.J.F. Reaction of meso-tetraarylporphyrins with pyrazine ortho-quinodimethanes. Tetrahedron Lett. 2005;46:2189–2191. doi: 10.1016/j.tetlet.2005.02.041. [DOI] [Google Scholar]
- 21.Silva A.M.G., Oliveira K.T., Faustino M.A.F., Neves M.G.P.M.S., Tomé A.C., Silva A.M.S., Cavaleiro J.A.S., Brandão P., Felix V. Chemical transformations of mono- and bis(buta-1,3-dien-1-yl)porphyrins: A new synthetic approach to mono- and dibenzoporphyrins. Eur. J. Org. Chem. 2008:704–712. doi: 10.1002/ejoc.200700852. [DOI] [Google Scholar]
- 22.Silva A.M.G., Tomé A.C., Neves M.G.P.M.S., Silva A.M.S., Cavaleiro J.A.S. meso-Tetraarylporphyrins as dipolarophiles in 1,3-dipolar cycloaddition reactions. Chem. Commun. 1999:1767–1768. doi: 10.1039/a905016g. [DOI] [Google Scholar]
- 23.Aguiar A., Leite A., Silva A.M.N., Tomé A.C., Cunha-Silva L., Castro B., Rangel M., Silva A.M.G. Isoxazolidine-fused meso-tetraarylchlorins as key tools for the synthesis of mono- and bis-annulated chlorins. Org. Biomol. Chem. 2015;13:7131–7135. doi: 10.1039/C5OB00800J. [DOI] [PubMed] [Google Scholar]
- 24.Silva A.M.G., Tomé A.C., Neves M.G.P.M.S., Silva A.M.S., Cavaleiro J.A.S. 1,3-Dipolar cycloaddition reactions of porphyrins with azomethine ylides. J. Org. Chem. 2005;70:2306–2314. doi: 10.1021/jo048349i. [DOI] [PubMed] [Google Scholar]
- 25.Silva A.M.G., Tomé A.C., Neves M.G.P.M.S., Cavaleiro J.A.S., Perrone D., Dondoni A. Porphyrins in 1,3-dipolar cycloadditions with sugar azomethine ylides. Synthesis of pyrrolidinoporphyrin glycoconjugates. Synlett. 2005:857–859. doi: 10.1002/chin.200531123. [DOI] [Google Scholar]
- 26.Vinhado F.S., Gandini M.E.F., Iamamoto Y., Silva A.M.G., Simões M.M.Q., Neves M.G.P.M.S., Tomé A.C., Rebelo S.L.H., Pereira A.M.V.M., Cavaleiro J.A.S. Novel Mn(III)chlorins as versatile catalysts for oxyfunctionalisation of hydrocarbons under homogeneous conditions. J. Mol. Catal. A Chem. 2005;239:138–143. doi: 10.1016/j.molcata.2005.06.014. [DOI] [Google Scholar]
- 27.Galęzowski M., Gryko D.T. Synthesis of locked meso-β-substituted chlorins via 1,3-dipolar cycloaddition. J. Org. Chem. 2006;71:5942–5950. doi: 10.1021/jo060545x. [DOI] [PubMed] [Google Scholar]
- 28.Silva A.M.G., Tomé A.C., Neves M.G.P.M.S., Cavaleiro J.A.S. Porphyrins in 1,3-dipolar cycloaddition reactions: Synthesis of a novel pyrazoline-fused chlorin and a pyrazole-fused porphyrin. Synlett. 2002;71:1155–1157. doi: 10.1055/s-2002-32581. [DOI] [Google Scholar]
- 29.Silva A.M.G., Tomé A.C., Neves M.G.P.M.S., Silva A.M.S., Cavaleiro J.A.S., Perrone D., Dondoni A. Porphyrins in 1,3-dipolar cycloaddition reactions with sugar nitrones. Synthesis of glycoconjugated isoxazolidine-fused chlorins and bacteriochlorins. Tetrahedron Lett. 2002;43:603–605. doi: 10.1016/S0040-4039(01)02243-2. [DOI] [Google Scholar]
- 30.Flemming J., Dolphin D. Carbonyl ylide 1,3-dipolar cycloadditions with porphyrins. Tetrahedron Lett. 2002;43:7281–7283. doi: 10.1016/S0040-4039(02)01619-2. [DOI] [Google Scholar]
- 31.Silva A.M.G., Tomé A.C., Neves M.G.P.M.S., Silva A.M.S., Cavaleiro J.A.S. Synthesis of new β-substituted meso-tetraphenylporphyrins via 1,3-dipolar cycloaddition reactions. 1. J. Org. Chem. 2002;67:726–732. doi: 10.1021/jo0106703. [DOI] [PubMed] [Google Scholar]
- 32.Akhigbe J., Samankumara L.P., Brückner C. The breaking and mending of porphyrins: Reductive coupling of secochlorin bisaldehydes. Tetrahedron Lett. 2012;53:3524–3526. doi: 10.1016/j.tetlet.2012.04.137. [DOI] [Google Scholar]
- 33.Boerner L.J.K., Dye D.F., Köpke T., Zaleski J.M. Expansion and contraction: Shaping the porphyrin boundary via diradical reactivity. Coord. Chem. Rev. 2013;257:599–620. doi: 10.1016/j.ccr.2012.07.009. [DOI] [Google Scholar]
- 34.Brückner C., Akhigbe J., Samankumara L. Porphyrin analogs containing non-pyrrolic heterocycles. In: Kadish K.M., Smith K.M., Guilard R., editors. Handbook of Porphyrin Science. volume 31. World Scientific; River Edge, NY, USA: 2014. pp. 1–276. [Google Scholar]
- 35.Callot H.J., Schaeffer E. Homologation directe du cycle des porphyrines par les diazoalcanes. Tetrahedron. 1978;34:2295–2300. doi: 10.1016/0040-4020(78)89041-3. [DOI] [Google Scholar]
- 36.Callot H.J. Ring expansions and contractions of metalloporphyrins. Dalton Trans. 2008:6346–6357. doi: 10.1039/b807066k. [DOI] [PubMed] [Google Scholar]
- 37.Crossley M.J., King L.G. Novel heterocyclic systems from selective oxidation at the β-pyrrolic position of porphyrins. J. Chem. Soc. Chem. Commun. 1984:920–922. doi: 10.1039/C39840000920. [DOI] [Google Scholar]
- 38.Crossley M.J., Hambley T.W., King L.G. Conversion of a porphyrin into a 5,6-dihydroporphyrin. Synthesis and X-ray crystal structure of (5RS,6SR)-5,6-dihydro-6-(methoxycarbonyl)-8-oxo-5,10,15,20-tetraphenyl-8H-7-oxaporphyrin. Bull. Soc. Chim. Fr. 1996;133:735–742. [Google Scholar]
- 39.Jayaraj K., Gold A., Austin R.N., Ball L.M., Terner J., Mandon D., Weiss R., Fischer J., DeCian A., Bill E., et al. Compound I and compound II analogues from porpholactones. Inorg. Chem. 1997;36:4555–4566. doi: 10.1021/ic970597s. [DOI] [PubMed] [Google Scholar]
- 40.Adams K.R., Bonnett R., Burke P.J., Salgado A., Vallés M.A. The 2,3-secochlorin-2,3-dione system. J. Chem. Soc. Chem. Commun. 1993:1860–1861. doi: 10.1039/c39930001860. [DOI] [Google Scholar]
- 41.Adams K.R., Bonnett R., Burke P.J., Salgado A., Vallés M.A. Cleavage of (octaethyl-2,3-dihydroxychlorinato)nickel(II) to give the novel 2,3-dioxo-2,3-secochlorin system. J. Chem. Soc. Perkin Trans. 1. 1997:1769–1772. doi: 10.1039/a701152k. [DOI] [Google Scholar]
- 42.Secochlorins are porphyrin derivatives in which a β,β′-bond was cleaved; chlorophins and bacteriophins are porphyrin derivatives in which one or two sets of β,β′-carbon atoms were removed
- 43.Ryppa C., Niedzwiedzki D., Morozowich N.L., Srikanth R., Zeller M., Frank H.A., Brückner C. Stepwise conversion of two pyrrole moieties of octaethylporphyrin to pyridin-3-ones: Synthesis, mass spectral, and photophysical properties of mono and bis(oxypyri)porphyrins. Chem. Eur. J. 2009;15:5749–5762. doi: 10.1002/chem.200900280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee S., Zeller M., Brückner C. meso‑Tetraphenylporphyrin-derived oxypyriporphyrin, oxypyrichlorin, and thiomorpholinochlorin, as their Ni(II) complexes. J. Porphyr. Phthalocyanines. 2012;16:576–588. doi: 10.1142/S1088424612500654. [DOI] [Google Scholar]
- 45.Brückner C., Rettig S.J., Dolphin D. Formation of a meso-tetraphenylsecochlorin and a homoporphyrin with a twist. J. Org. Chem. 1998;63:2094–2098. doi: 10.1021/jo971156t. [DOI] [Google Scholar]
- 46.Brückner C., Sternberg E.D., MacAlpine J.K., Rettig S.J., Dolphin D. A novel stepwise degradation of porphyrins. Synthesis and structural characterization of meso-tetraphenylchlorophinato nickel(II) and meso-tetraphenylsecochlorinato nickel(II) J. Am. Chem. Soc. 1999;121:2609–2610. doi: 10.1021/ja981417w. [DOI] [Google Scholar]
- 47.Brückner C., Dolphin D. 2,3-vic-Dihydroxy-meso-tetraphenylchlorins from the osmium tetroxide oxidation of meso-tetraphenylporphyrin. Tetrahedron Lett. 1995;36:3295–3298. doi: 10.1016/0040-4039(95)00524-G. [DOI] [Google Scholar]
- 48.Brückner C., Dolphin D. β,β‘-Dihydroxylation of meso-tetraphenylchlorins and metallochlorins. Tetrahedron Lett. 1995;36:9425–9428. doi: 10.1016/0040-4039(95)02052-7. [DOI] [Google Scholar]
- 49.Daniell H.W., Brückner C. Enantiomeric resolution of a ruffled porphyrinoid. Angew. Chem. Int. Ed. 2004;43:1688–1691. doi: 10.1002/anie.200352970. [DOI] [PubMed] [Google Scholar]
- 50.Brückner C., Götz D.C.G., Fox S.P., Ryppa C., McCarthy J.R., Bruhn T., Akhigbe J., Banerjee S., Daddario P., Daniell H.W., et al. Helimeric porphyrinoids: Stereostructure and chiral resolution of meso-tetraarylmorpholinochlorins. J. Am. Chem. Soc. 2011;133:8740–8752. doi: 10.1021/ja202451t. [DOI] [PubMed] [Google Scholar]
- 51.Campbell C.J., Rusling J.F., Brückner C. Nickel(II) meso-tetraphenyl-homoporphyrins, -secochlorins, and -chlorophin: Control of redox chemistry by macrocycle rigidity. J. Am. Chem. Soc. 2000;122:6679–6685. doi: 10.1021/ja000749+. [DOI] [Google Scholar]
- 52.McCarthy J.R., Jenkins H.A., Brückner C. Free base meso-tetraaryl-morpholinochlorins and porpholactone from meso-tetraaryl-2,3-dihydroxy-chlorin. Org. Lett. 2003;5:19–22. doi: 10.1021/ol027072a. [DOI] [PubMed] [Google Scholar]
- 53.Khalil G.E., Daddario P., Lau K.S.F., Imtiaz S., King M., Gouterman M., Sidelev A., Puran N., Ghandehari M., Brückner C. meso-Tetraarylporpholactones as high pH sensors. Analyst. 2010;135:2125–2131. doi: 10.1039/c0an00018c. [DOI] [PubMed] [Google Scholar]
- 54.Brückner C., Ogikubo J., McCarthy J.R., Akhigbe J., Hyland M.A., Daddario P., Worlinsky J.L., Zeller M., Engle J.T., Ziegler C.J., et al. meso-Arylporpholactones and their reduction products. J. Org. Chem. 2012;77:6480–6494. doi: 10.1021/jo300963m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banerjee S., Zeller M., Brückner C. OsO4-mediated dihydroxylation of meso-tetraphenylporphyrin N-oxide and transformation of the resulting diolchlorin N-oxide regioisomers. J. Org. Chem. 2010;75:1179–1187. doi: 10.1021/jo9024286. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy J.R., Melfi P.J., Capetta S.H., Brückner C. Use of Ag(II) as a removable template in porphyrin chemistry: Diol cleavage products of [meso-tetraphenyl-2,3-cis-diolchlorinato]silver(II) Tetrahedron. 2003;59:9137–9146. doi: 10.1016/j.tet.2003.09.067. [DOI] [Google Scholar]
- 57.Lara K.K., Rinaldo C.R., Brückner C. meso-Tetraaryl-7,8-dihydroxydithiachlorins: First examples of heterochlorins. Tetrahedron Lett. 2003;44:7793–7797. doi: 10.1016/j.tetlet.2003.08.070. [DOI] [Google Scholar]
- 58.Lara K.K., Rinaldo C.K., Brückner C. meso-Tetraaryl-7,8-diol-21,23-dithiachlorins and their pyrrole-modified derivatives: A spectroscopic comparison to their aza-analogues. Tetrahedron. 2005;61:2529–2539. doi: 10.1016/j.tet.2004.12.043. [DOI] [Google Scholar]
- 59.Akhigbe J., Ryppa C., Zeller M., Brückner C. Oxazolochlorins. 2. Intramolecular Cannizzaro reaction of meso-tetraphenylsecochlorin bisaldehyde. J. Org. Chem. 2009;74:4927–4933. doi: 10.1021/jo9006046. [DOI] [PubMed] [Google Scholar]
- 60.Samankumara L.P., Wells S., Zeller M., Acuña A.M., Röder B., Brückner C. Expanded bacteriochlorins. Angew. Chem. Int. Ed. 2012;51:5757–5760. doi: 10.1002/anie.201201124. [DOI] [PubMed] [Google Scholar]
- 61.Samankumara L.P., Zeller M., Krause J.A., Brückner C. Syntheses, structures, modification, and optical properties of meso-tetraaryl-2,3-dimethoxychlorin, and two isomeric meso-tetraaryl-2,3,12,13-tetrahydroxybacteriochlorins. Org. Biomol. Chem. 2010;8:1951–1965. doi: 10.1039/b924539a. [DOI] [PubMed] [Google Scholar]
- 62.McCarthy J.R., Hyland M.A., Brückner C. Indaphyrin, a meso-tetraphenylsecochlorin-derived chromophore incorporating o-phenyl-to-β-linkages. Chem. Commun. 2003:1738–1739. doi: 10.1039/B304647H. [DOI] [Google Scholar]
- 63.McCarthy J.R., Hyland M.A., Brückner C. Synthesis of indaphyrins: Meso-Tetraarylsecochlorin-based porphyrinoids containing direct o-phenyl-to-β-linkages. Org. Biomol. Chem. 2004;2:1484–1491. doi: 10.1039/B401629G. [DOI] [PubMed] [Google Scholar]
- 64.Lau K.S.F., Zhao S., Ryppa C., Jockusch S., Turro N.J., Zeller M., Gouterman M., Khalil G.E., Brückner C. Synthesis, structure, and optical properties of the platinum(II) complexes of indaphyrin and thiaindaphyrin. Inorg. Chem. 2009;48:4067–4074. doi: 10.1021/ic802041z. [DOI] [PubMed] [Google Scholar]
- 65.Samankumara L.P., Dorazio S.J., Akhigbe J., Li R., Nimthong-Roldán A., Zeller M., Brückner C. Indachlorins: Nonplanar indanone-annulated chlorin analogues with panchromatic absorption spectra between 300 and 900 nm. Chem. Eur. J. 2015;21:11118–11128. doi: 10.1002/chem.201501230. [DOI] [PubMed] [Google Scholar]
- 66.Götz D.C.G., Gehrold A.C., Dorazio S.J., Daddario P., Samankumara L., Bringmann G., Brückner C., Bruhn T. Indaphyrins and indachlorins: Optical and chiroptical properties of a family of helimeric porphyrinoids. Eur. J. Org. Chem. 2015;18:3913–3922. doi: 10.1002/ejoc.201500511. [DOI] [Google Scholar]
- 67.Banerjee S., Hyland M.A., Brückner C. [2-Methylazeteochlorinato]Ni(II): A pyrrole ring-contracted chlorin analogue. Tetrahedron Lett. 2010;51:4505–4508. doi: 10.1016/j.tetlet.2010.06.096. [DOI] [Google Scholar]
- 68.Brückner C., Hyland M.A., Sternberg E.D., MacAlpine J.K., Rettig S.J., Patrick B.O., Dolphin D. Preparation of [meso-tetraphenylchlorophinato]nickel(II) by stepwise deformylation of [meso-tetraphenyl-2,3-diformyl-secochlorinato]nickel(II): Conformational consequences of breaking the structural integrity of nickel porphyrins. Inorg. Chim. Acta. 2005;358:2943–2953. doi: 10.1016/j.ica.2004.09.015. [DOI] [Google Scholar]
- 69.Köpke T., Pink M., Zaleski J.M. Elucidation of the extraordinary 4-membered pyrrole ring-contracted azeteoporphyrinoid as an intermediate in chlorin oxidation. Chem. Commun. 2006:4940–4942. doi: 10.1039/B611567E. [DOI] [PubMed] [Google Scholar]
- 70.Banerjee S., Zeller M., Brückner C. MTO/H2O2/pyrazole-mediated N-oxidation of meso-tetraarylporphyrins and -chlorins, and S-oxidation of a meso-tetraaryldithiaporphyrin and -chlorin. J. Org. Chem. 2009;74:4283–4288. doi: 10.1021/jo9005443. [DOI] [PubMed] [Google Scholar]
- 71.Kozyrev A.N., Alderfer J.L., Dougherty T.J., Pandey R.K. Synthesis of mono- and di(oxopyri)porphyrins: a new approach through ring enlargement with diazomethane. Angew. Chem. Int. Ed. 1999;38:126–128. doi: 10.1002/(SICI)1521-3773(19990115)38:1/2<126::AID-ANIE126>3.0.CO;2-U. [DOI] [Google Scholar]
- 72.Akhigbe J., Brückner C. Expansion of a pyrrole in meso-tetraphenylporphyrin to a pyrazine imide moiety using a Beckmann rearrangement. Eur. J. Org. Chem. 2013:3876–3884. doi: 10.1002/ejoc.201300274. [DOI] [Google Scholar]
- 73.Akhigbe J., Zeller M., Brückner C. Quinoline-annulated porphyrins. Org. Lett. 2011;13:1322–1325. doi: 10.1021/ol1031848. [DOI] [PubMed] [Google Scholar]
- 74.Akhigbe J., Luciano M., Zeller M., Brückner C. Mono- and bisquinoline-annulated porphyrins from porphyrin β,β′‑dione oximes. J. Org. Chem. 2015;80:499–511. doi: 10.1021/jo502511j. [DOI] [PubMed] [Google Scholar]
- 75.Cavaleiro J.A.S., Gerdan V.M., Hombrecher H.K., Neves M.G.P.M.S., Silva A.M.S. Synthesis and characterisation of new 2-diazo-3-oxo-5,10,15,20-tetraphenylchlorins. Heterocycl. Commun. 1997;3:253–261. doi: 10.1515/HC.1997.3.3.253. [DOI] [Google Scholar]
- 76.Köpke T., Pink M., Zaleski J.M. A facile synthesis of 2-diazo-3-oxochlorins by lewis acid activation: selective modification of π-electron conjugated macrocycles. Synlett. 2006:2183–2186. doi: 10.1055/s-2006-949613. [DOI] [Google Scholar]
- 77.Hombrecher H.K., Gerdan V.M., Cavaleiro J.A.S., Neves M.G.P.M.S. Photoinduced reaction of 2-diazo-3-oxo-5,10,15,20-tetraphenylchlorins with alcohols. Heterocycl. Commun. 1997;3:453–460. doi: 10.1515/HC.1997.3.5.453. [DOI] [Google Scholar]
- 78.Köpke T., Pink M., Zaleski J.M. Photochemical preparation of pyrrole ring-contracted chlorins by the Wolff rearrangement. Org. Biomol. Chem. 2006;4:4059–4062. doi: 10.1039/B612776M. [DOI] [PubMed] [Google Scholar]
- 79.Köpke T., Pink M., Zaleski J.M. Expansion by contraction: Diversifying the photochemical reactivity scope of diazo-oxochlorins toward development of in situ alkylating agents. J. Am. Chem. Soc. 2008;130:15864–15871. doi: 10.1021/ja800094e. [DOI] [PubMed] [Google Scholar]
- 80.Kopke T., Zaleski J.M. Diazo-containing molecular constructs as potential anticancer agents: From diazo[b]fluorene natural products to photoactivatable diazo-oxochlorins. Anti-Cancer Agents Med. Chem. 2008;8:292–304. doi: 10.2174/187152008783961941. [DOI] [PubMed] [Google Scholar]
- 81.Dye D.F., Köpke T., Ramabhadran R.O., Raghavachari K., Zaleski J.M. Gating the mechanistic pathway to the elusive 4-membered ring azeteoporphyrin. J. Am. Chem. Soc. 2011;133:13110–13120. doi: 10.1021/ja203451k. [DOI] [PubMed] [Google Scholar]
- 82.Meehan E., Li R., Zeller M., Brückner C. Octaethyl-1,3-oxazinochlorin: A β‑octaethylchlorin analogue made by pyrrole expansion. Org. Lett. 2015;17:2210–2213. doi: 10.1021/acs.orglett.5b00800. [DOI] [PubMed] [Google Scholar]
- 83.Li K.-L., Guo C.-C., Chen Q.-Y. Unprecedented degradation of nickel(II) 2,3,12,13-tetrabromo-5,10,15,20-tetraarylporphyrins by the anion of E-benzaldoxime: A novel approach to nickel(II) chlorophins and bacteriophins. Org. Lett. 2010;11:2724–2727. doi: 10.1021/ol901052w. [DOI] [PubMed] [Google Scholar]
- 84.Shulga A.M., Biteva I.M., Gurinovich I.F., Grubina L.A., Gurinovich G.P. Oxidation of porphyrins—Structure of products of octaethylporphin ozonization. Biofisika. 1977;22:771–776. [PubMed] [Google Scholar]
- 85.Gouterman M., Hall R.J., Khalil G.-E., Martin P.C., Shankland E.G., Cerny R.L. Tetra(pentafluorophenyl)porpholactone. J. Am. Chem. Soc. 1989;111:3702–3707. doi: 10.1021/ja00192a030. [DOI] [Google Scholar]
- 86.Khalil G., Gouterman M., Ching S., Costin C., Coyle L., Gouin S., Green E., Sadilek M., Wan R., Yearyean J., et al. Synthesis and spectroscopic characterization of Ni, Zn, Pd and Pt tetra(pentafluorophenyl)porpholactone with comparisons to Mg, Zn, Y, Pd and Pt metal complexes of tetra(pentafluorophenyl)porphine. J. Porphyr. Phthalocyanines. 2002;6:135–145. doi: 10.1142/S108842460200018X. [DOI] [Google Scholar]
- 87.Lv H., Yang B., Jing J., Yu Y., Zhang J., Zhang J.-L. Dual facet of gold(III) in the reactions of gold(III) and porphyrins. Dalton Trans. 2012;41:3116–3118. doi: 10.1039/c2dt12280d. [DOI] [PubMed] [Google Scholar]
- 88.Yu Y., Lv H., Ke X., Yang B., Zhang J.-L. Ruthenium-catalyzed oxidation of the porphyrin β-β’-pyrrolic ring: A general and efficient approach to porpholactones. Adv. Synth. Catal. 2012;354:3509–3516. doi: 10.1002/adsc.201200720. [DOI] [Google Scholar]
- 89.Ke X.-S., Chang Y., Chen J.-Z., Tian J., Mack J., Cheng X., Shen Z., Zhang J.-L. Porphodilactones as synthetic chlorophylls: Relative orientation of β‑substituents on a pyrrolic ring tunes NIR absorption. J. Am. Chem. Soc. 2014;136:9598–9607. doi: 10.1021/ja502729x. [DOI] [PubMed] [Google Scholar]
- 90.Akhigbe J., Peters G., Zeller M., Brückner C. Unexpected hydroxylamine-induced ring-closure reactions of meso-tetraphenylsecochlorin bisaldehyde. Org. Biomol. Chem. 2011;9:2306–2313. doi: 10.1039/c0ob00920b. [DOI] [PubMed] [Google Scholar]
- 91.Akhigbe J., Haskoor J., Zeller M., Brückner C. Porpholactams and chlorolactams: Replacement of a β-β’-double bond in meso-tetraphenyl-porphyrin and -chlorin by a lactam moiety. Chem. Commun. 2011;47:8599–8601. doi: 10.1039/c1cc12955d. [DOI] [PubMed] [Google Scholar]
- 92.Akhigbe J., Haskoor J., Krause J.A., Zeller M., Brückner C. Formation, structure, and reactivity of meso-tetraarylchlorolactones, -porpholactams, and -chlorolactams, porphyrin and chlorin analogues incorporating oxazolone or imidazolone moieties. Org. Biomol. Chem. 2013;11:3616–3628. doi: 10.1039/c3ob40138c. [DOI] [PubMed] [Google Scholar]
- 93.Brückner C., McCarthy J.R., Daniell H.W., Pendon Z.D., Ilagan R.P., Francis T.M., Ren L., Birge R.R., Frank H.A. A spectroscopic and computational study of the singlet and triplet excited states of synthetic β-functionalized chlorins. Chem. Phys. 2003;294:285–303. doi: 10.1016/S0301-0104(03)00282-9. [DOI] [Google Scholar]
- 94.McCarthy J.R., Perez M.J., Brückner C., Weissleder R. Polymeric nanoparticle preparation that eradicates tumors. Nano Lett. 2005;5:2552–2556. doi: 10.1021/nl0519229. [DOI] [PubMed] [Google Scholar]
- 95.Ogikubo J., Worlinsky J.L., Fu Y.-J., Brückner C. A two-step, one-pot route to swap the pyrroline moiety in meso-tetraaryldihydroxy-chlorins with an O/N-substituted oxazoline. Tetrahedron Lett. 2013;54:1707–1710. doi: 10.1016/j.tetlet.2013.01.070. [DOI] [Google Scholar]
- 96.Ogikubo J., Brückner C. Tunable meso-tetraphenyl-alkyloxazolo-chlorins and –bacteriochlorins. Org. Lett. 2011;13:2380–2383. doi: 10.1021/ol2006264. [DOI] [PubMed] [Google Scholar]
- 97.Ogikubo J., Meehan E., Engle J.T., Ziegler C.J., Brückner C. meso-Aryl-3-alkyl-2-oxachlorins. J. Org. Chem. 2012;77:6199–6207. doi: 10.1021/jo300964v. [DOI] [PubMed] [Google Scholar]
- 98.Ogikubo J., Meehan E., Engle J.T., Ziegler C.J., Brückner C. Oxazolochlorins. 9. meso-Tetraphenyl-2-oxabacteriochlorins and meso-tetraphenyl-2,12/13-dioxabacteriochlorins. J. Org. Chem. 2013;78:2840–2852. doi: 10.1021/jo400031r. [DOI] [PubMed] [Google Scholar]
- 99.Yu Y., Czepukojc B., Jacob C., Jiang Y., Zeller M., Brückner C., Zhang J.-L. Porphothionolactones: Synthesis, structure, physical, and chemical properties of a chemodosimeter for hypochlorite. Org. Biomol. Chem. 2013;11:4613–4621. doi: 10.1039/c3ob40758f. [DOI] [PubMed] [Google Scholar]
- 100.Head M.L., Zarate G., Brückner C. Pyrazinoporphyrins: Expanding a pyrrolic building block in meso‑tetraphenylporphyrin by a nitrogen atom. Eur. J. Org. Chem. 2016:992–998. doi: 10.1002/ejoc.201501436. [DOI] [Google Scholar]
- 101.Ke X.-S., Tang J., Chen J.-J., Zhou Z.-Y., Zhang J.-L. β-Ionic conjugated chlorin-type photosensitizers based on porpholactone: Synthesis, photophysical properties, and photodynamic activity. ChemPlusChem. 2015;80:237–252. doi: 10.1002/cplu.201402356. [DOI] [Google Scholar]
- 102.Tang J., Chen J.-J., Jing J., Chen J.-Z., Lv H., Yu Y., Xu P., Zhang J.-L. β-Lactonization of fluorinated porphyrin enhances LDL binding affinity, cellular uptake with selective intracellular localization. Chem. Sci. 2014;5:558–566. doi: 10.1039/C3SC52247D. [DOI] [Google Scholar]
- 103.Ke X.-S., Tang J., Yang Z.-S., Zhang J.-L. β-Conjugation of gadolinium(III) DOTA complexes to zinc(II) porpholactol as potential multimodal imaging contrast agents. J. Porphyr. Phthalocyanines. 2014;18:950–959. doi: 10.1142/S1088424614500758. [DOI] [Google Scholar]
- 104.Cetin A., Ziegler C.J. Structure and catalytic activity of a manganese(III) tetraphenylporpholactone. Dalton Trans. 2005:25–26. doi: 10.1039/B417321J. [DOI] [PubMed] [Google Scholar]
- 105.Rahimi R., Tehrani A.A., Fard M.A., Sadegh B.M.M., Khavasi H.R. First catalytic application of metal complexes of porpholactone and dihydroxychlorin in the sulfoxidation reaction. Catal. Commun. 2009;11:232–235. doi: 10.1016/j.catcom.2009.09.022. [DOI] [Google Scholar]
- 106.Liang L., Lv H., Yu Y., Wang P., Zhang J.-L. Iron(III) tetrakis(pentafluorophenyl)porpholactone catalyzes nitrogen atom transfer to C=C and C–H bonds with organic azides. Dalton Trans. 2012;41:1457. doi: 10.1039/C2DT11995A. [DOI] [PubMed] [Google Scholar]
- 107.To W.-P., Liu Y., Lau T.-C., Che C.-M. A robust palladium(II)–porphyrin complex as catalyst for visible light induced oxidative C–H functionalization. Chem. Eur. J. 2013;19:5654–5664. doi: 10.1002/chem.201203774. [DOI] [PubMed] [Google Scholar]
- 108.Ishihara S., Labuta J., van Rossom W., Ishikawa D., Minami K., Hill J.P., Ariga K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014;16:9713–9746. doi: 10.1039/c3cp55431g. [DOI] [PubMed] [Google Scholar]
- 109.Zelelow B., Khalil G.E., Phelan G., Carlson B., Gouterman M., Callis J.B., Dalton L.R. Dual luminophor pressure sensitive paint. II. Lifetime based measurement of pressure and temperature. Sens. Actuators B. 2003;96:304–314. doi: 10.1016/S0925-4005(03)00547-1. [DOI] [Google Scholar]
- 110.Khalil G.E., Costin C., Crafton J., Jones G., Grenoble S., Gouterman M., Callis J.B., Dalton L.R. Dual-luminophor pressure-sensitive paint. I. Ratio of reference to sensor giving a small temperature dependency. Sens. Actuators B. 2004;97:13–21. doi: 10.1016/S0925-4005(03)00484-2. [DOI] [Google Scholar]
- 111.Gouterman M., Callis J., Dalton L., Khalil G., Mébarki Y., Cooper K.R., Grenier M. Dual luminophor pressure-sensitive paint: III. Application to automotive model testing. Meas. Sci. Technol. 2004;15:1986–1994. doi: 10.1088/0957-0233/15/10/007. [DOI] [Google Scholar]
- 112.Waskitoaji W., Hyakutake T., Watanabe M., Nishide H. Pt-porpholactone- and -porphyrin-based luminescent sensory polymer coating for visualization of oxygen pressure distribution on biplanar surface. React. Funct. Polym. 2010;70:669–673. doi: 10.1016/j.reactfunctpolym.2010.05.013. [DOI] [Google Scholar]
- 113.Ishigami Y., Waskitoaji W., Yoneda M., Takada K., Hyakutake T., Suga T., Uchida M., Nagumo Y., Inukai J., Nishide H., et al. Oxygen partial pressures on gas-diffusion layer surface and gas-flow channel wall in polymer electrolyte fuel cell during power generation studied by visualization technique combined with numerical simulation. J. Power Sources. 2014;269:556–564. doi: 10.1016/j.jpowsour.2014.07.017. [DOI] [Google Scholar]
- 114.Rodrigues J.M.M., Farinha A.A.S., Tomé A.C., Cavaleiro J.A.S., Tomé J.P.C. Highly selective optical chemosensor for cyanide in aqueous medium. Sens. Actuators B Chem. 2016;224:81–87. doi: 10.1016/j.snb.2015.10.026. [DOI] [Google Scholar]
- 115.Worlinsky J.L., Halepas S., Brückner C. PEGylated meso-arylporpholactone metal complexes as optical cyanide sensors in water. Org. Biomol. Chem. 2014;12:3991–4001. doi: 10.1039/C4OB00697F. [DOI] [PubMed] [Google Scholar]
- 116.Ke X.-S., Yang B.-Y., Cheng X., Chan S.L.-F., Zhang J.-L. Ytterbium(III) porpholactones: β-Lactonization of porphyrin ligands enhances sensitization efficiency of lanthanide near-infrared luminescence. Chem. Eur. J. 2014;20:4324–4333. doi: 10.1002/chem.201303972. [DOI] [PubMed] [Google Scholar]
- 117.Worlinsky J.L., Zarate G., Zeller M., Ghandehari M., Khalil G., Brückner C. Oxazolochlorins. 11. Tuning the dynamic high pH sensing range of [meso-tetraarylporpholactonato]M(II) complexes by variation of the central metal ion, the aryl substituents, and introduction of a β-nitro group. J. Porphyr. Phthalocyanines. 2013;17:836–849. doi: 10.1142/S1088424613500478. [DOI] [Google Scholar]
- 118.Worlinsky J.L., Halepas S., Ghandehari M., Khalil G., Brückner C. High pH sensing with water-soluble porpholactone derivatives and their incorporation into a Nafion® optode membrane. Analyst. 2015;140:190–196. doi: 10.1039/C4AN01462F. [DOI] [PubMed] [Google Scholar]