Abstract
The soft coral genus Sinularia is a rich source of bioactive metabolites containing a diverse array of chemical structures. A solvent extract of Sinularia polydactyla resulted in the isolation of three new casbane diterpenes: sinularcasbane M (1), sinularcasbane N (2) and sinularcasbane O (3); in addition, known metabolites (4–5) were isolated. Compounds were elucidated on the basis of spectroscopic analyses; the absolute configuration was confirmed by X-ray analysis.
Keywords: soft coral, alcyoniidae, Sinularia polydactyla, diterpenes
1. Introduction
In Alcyonacean soft coral, the genus Sinularia is a rich source of diverse natural products with over 500 metabolites including sesquiterpenes, diterpenes, polyhydroxylated steroids, alkaloids and polyamines already having been chemically characterized [1,2,3,4,5]. Sinularia consists of almost 90 species of which approximately 50 have been profiled for biological activity; such studies have established that the genus is a rich source of secondary metabolites with biological properties including cytotoxic, anti-inflammatory and antimicrobial activities [5,6,7,8,9,10]. While Sinularia species occur in marine waters around the world, S. polydactylais (Figure 1) is endemic to the Red Sea. Although the marine environment contains extensive reef formations, this ecosystem is not as well characterized in terms of chemical studies of marine organisms compared to other large coral reef systems [11]. As a part of a comprehensive chemical inventory of marine natural products from Red Sea soft coral [12,13,14], herein, we reported the chemical characterization of solvent-extracted casbane diterpenes from S. polydactyla (Figure 2). Casbane-type diterpene structures are related to the 14-membered cembrane ring system except for the dimethyl-cyclopropyl moiety instead of an isopropyl residue fused to the ring. These extremely-rare natural products are predominantly isolated from select coral species [15]. Several soft coral casbanes have been isolated from Sinularia depressa with biologically active 10-hydroxydepressin exhibiting cytotoxicity against several tumor cell lines with IC50 values near 50 μM [16].
Figure 1.
Soft coral Sinularia polydactyla.
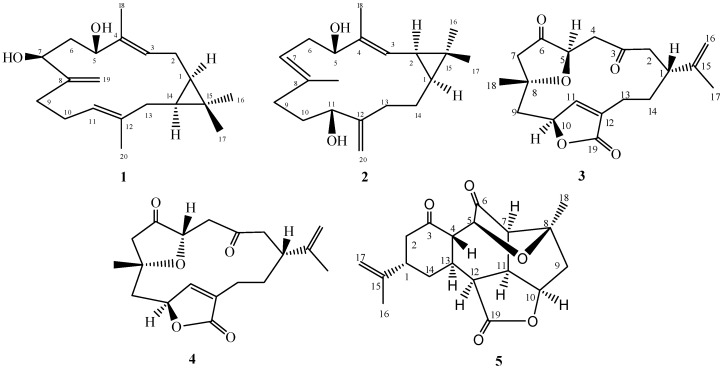
Figure 2.
Structures of metabolites 1–5.
2. Results and Discussion
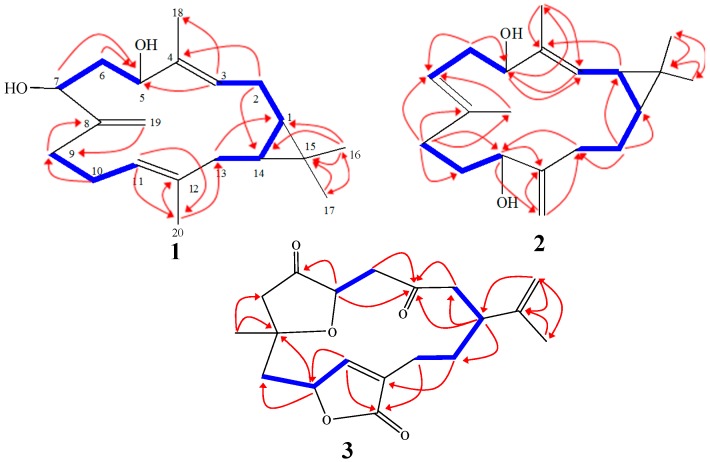
Compound 1 was obtained as a colorless oil with a negative optical rotation ( − 9.6 in MeOH). HR-ESI-FTMS analysis exhibited a molecular ion peak at m/z 327.2293 [M + Na]+ corresponding to a molecular formula of C20H32O2 (calcd. for C20H32O2Na, 327.2300) with five degrees of unsaturation. The IR spectrum exhibited bands for hydroxyl and olefinic substituents at 3409, and 1650 cm−l, respectively. The 1H-NMR spectrum showed four methyl signals, including two olefinic methyls at δH (1.56 brs and 1.74 brs, each 3H) and two tertiary methyls at δH (1.00 s and 1.06 s, each 3H). Olefinic protons at δH 5.02 (brd, J = 8.3 Hz) and 5.19 (brd, J = 8.3 Hz) were attributed to two trisubstituted double bonds; exomethylene singlets were observed at δH 4.89 and 5.04. In addition, two secondary oxygenated proton signals appeared at δH 3.93 (brd, J = 11.1 Hz) and 4.39 (dd, J = 10.0, 4.3 Hz). The 13C-NMR spectrum displayed 20 carbon resonances which were classified by DEPT spectra as four methyls, six methylenes, six methines and four quaternary carbons. In addition, two oxygenated carbons at δC 71.2 and 77.0 and six olefinic carbons at δC 125.4, 137.9, 124.4, 136.9, 154.0, and 109.8 were observed. A casbane-type diterpene skeleton was predicted based on NMR data comparisons of analogous structures previously isolated from the same genus [4,15,16]. Characteristic signals at δC 25.0, 31.5, and 20.1 were proposed to constitute a cyclopropane moiety assigned to C-1, C-14 and C-15, respectively by DQF-COSY and HMBC analyses. The DQF-COSY showed correlations between signals at δH 0.69 (td, J = 10.9, 2.6 Hz) and 0.67 (td, J = 10.9, 4.0 Hz) with methylene mutiplet signals δH 1.26 and 1.80/2.19, respectively. Additionally, HMBC data confirmed these carbon assignments with correlations with signals at δH 0.69 (td, J = 10.9 and 2.6 Hz, H-1), 0.67 (td, J = 10.9 and 4.0 Hz, H-14), 1.26 (m, H2-2) and 1.80/2.19 (m, H2-13). Two singlet signals at δC 15.8 and 28.7 were assigned to methyl groups at C-16 and C-17 respectively and attached to C-15 based on HMBC correlations (Figure 3). The olefinic signal at δH 5.19 (d, J = 8.3 Hz) was assigned to H-3 based on an observed DQF-COSY correlation with H-2 and HMBC correlation between H-3 and an olefinic methyl and oxygenated methine at δC 10.5 (C-18) and 77.0 (C-5), respectively. Additionally, HMBC correlations allowed for the assignment of H-3 (δH 5.19), H3-18 (δH 1.74), and H-5 (δH 4.39), (Figure 3). DQF-COSY correlations with H-5 allowed for a methylene signal assignment at δH 1.60 (ddd, J = 13.8, 10.0, 2.2 Hz, H-6)/1.94 (ddd, J = 13.8, 10.0, 2.2 Hz, H-6) as well as the oxygenated signal at δH 3.93 (brd, J = 11.1 Hz, H-7). C-7 (δC 71.2) exhibited a HMBC correlation with exocyclic broad singlets at δH 4.89 (H-19) and 5.04 (H-19). HMBC correlations were observed between C-19 and δH 2.07 (m, H-9)/2.19 (m, H-9) as well as between H-9 and δC 154 (C-8). DQF-COSY correlation between H-9 and δH 2.07/2.19 (m) and 5.02 (brd, J = 8.3 Hz) allowed for the assignments of H-10 and H-11, respectively and the endocyclic proton H-11 coupled with δC 40.7 (C-13) in the HMBC spectrum. Finally, HMBC correlations were observed between H-11 and δC 15.8 (C-20) as well as H-20 and endocyclic signal δC 136.0 (C-12).
Figure 3.
Selected 1H-1H COSY () and HMBC () correlations of 1–3.
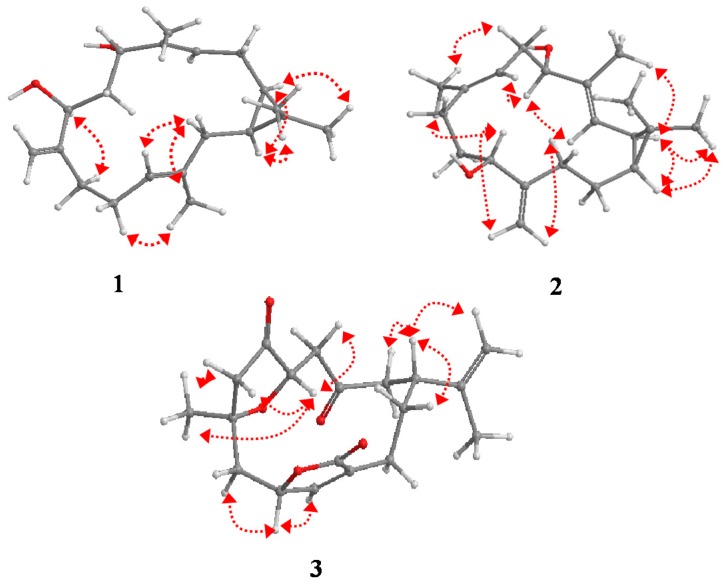
NOESY correlations observed between H-1, H-14 and H-16, indicated protons oriented on the same side consistent with a cis ring junction. The spectrum showed two proton signals attributed to 1,2-cyclopropane moiety at δH 0.69 (td, J = 10.9, 2.6 Hz, H-1) and 0.67 (td, J = 10.9, 4.0 Hz, H-14). Due to the flexible nature of the 14-membered macrocyclic ring, the relative configuration of the hydroxyl groups at C-5 and C-7 could not be determined by NOESY; however coupling constants for H-5/H-6a and H-6b/H-7 of 4.3 and 11.1 Hz respectively, indicated an cis-configuration for the hydroxyls. H-1α, H-3 and H-5 NOE correlations led to an assignment of H-5α and H-7α (Figure 4) which was confirmed by chemical shifts of δC 15.6 and 28.7 for C-16 and C-17 methyl groups respectively, and consistent with previously reported cis-fused casbane derivatives [4,15]. Based on this spectral analysis, 1 was identified as 5β,7β-dihydroxy-1α,14α-casba-3,8(19),11-triene, named here as a sinularcasbane M.
Figure 4.
Energy-minimized 3D structure and NOESY correlations () for 1–3.
Compound 2 was obtained as colorless crystals with a negative optical rotation ( − 15.5 in MeOH). HR-ESI-FTMS analysis showed a molecular ion peak at m/z 327.2295 [M + Na]+ corresponding to a molecular formula of C20H32O2 (calcd. for C20H32O2Na, 327.2300) with five degrees of unsaturation. The IR spectrum exhibited bands for hydroxyl and olefinic groups at 3409, and 1650 cm−l, respectively. Spectroscopic data was similar to 1 except for oxygenated and cyclopropane proton signals. The DQF-COSY spectrum showed a correlation between one of the cyclopropane signal δH 1.28 (dd, J = 10.1, 8.9 Hz) and the olefinic signal at δH 5.09 (dd, J = 10.1, 1.2 Hz) introducing the possibility of the cyclopropane ring or double bond shifting. The second cyclopropane signal at δH 0.77 (ddd, J = 11.9, 8.9, 2.7 Hz) correlates with the methylene protons at δH 1.13 (1H, dddd, J = 13.4, 12.8, 11.9, 3.4 Hz) and 1.55 (1H, dddd, J = 14.0, 13.4, 4.9, 2.7 Hz) which is consistent with the cyclopropane moiety being shifted to C-1 and C-2 and the olefinic bond remaining at C-3 and C-4. Also consistent with this shift, the olefinic proton H-3 correlates with an oxygenated signal at δC 79.5 in the HMBC spectrum allowing for a hydroxyl group to be located at C-5 (δH 3.97, dd, J = 10.7, 4.6 Hz). H-5 correlated with the methylene signals at δH 2.31 (ddd, J = 13.4, 10.7, 7.9 Hz)/2.38 (brdd, J = 13.4, 4.6 Hz) in DQF-COSY, allowing for the assignment of H-6. H-6 showed a correlation with the olefinic methine signal at δH 4.86 (brdd, J = 7.9, 1.2 Hz, H-7) as well as the broad methyl singlet at δH 1.63 (H3-19) in DQF-COSY and HMBC, respectively. Similar spectra for 1 and 2 allowed for the exomethylene to be assigned to C-12/C-20. HMBC correlation between C-12 and δH 3.90 (dd, 7.6, 5.2 Hz, H-11) as well DQF-COSY correlations between H-11 and δH 1.52/1.66 (m, H-10) and H-10 with δH 1.98 (dd, 15.6, 7.6, H-9)/2.09 (ddd, 15.6, 13.1, 3.1, H-9) established methylene groups at C-9-C-10 and a hydroxyl at C-11. A HMBC correlation between C-20 and δH 1.66 (m, H-13)/2.14 (ddd, 12.8, 12.8, 4.9, H-13) along with DQF-COSY correlations between H-13 and δH 1.13 (dddd, 13.4, 12.8, 11.9, 3.4, H-14)/1.55 (dddd, 14.0, 13.4, 4.9, 2.7, H-14), δH 0.77 (ddd, 11.9, 8.9, 2.7, H-1) and δH 1.28 (dd, 10.1, 8.9, H-2) allowed for the assignment of the other NMR signals (Figure 3). The relative configuration of hydroxyl group at C-11 was based on biogenetic considerations of casbane derivatives published by Yin, et al. in 2013, Yang, et al., 2015, and Li, et al. in 2010 [4,15,16], which was supported by coupling constant and NOESY experiments to be in the β position. Consequently, the relative configurations of H-1 and H-2 showed interaction with H-16 in NOESY experiments to be in the α position (Figure 3). Absolute configuration was established by X-ray crystallography (Figure 5). Therefore, 2 was assigned as 5S,11R-dihydroxy-1S,2R-casba-3,7,12(20)-triene, a new natural product named here as sinularcasbane N.
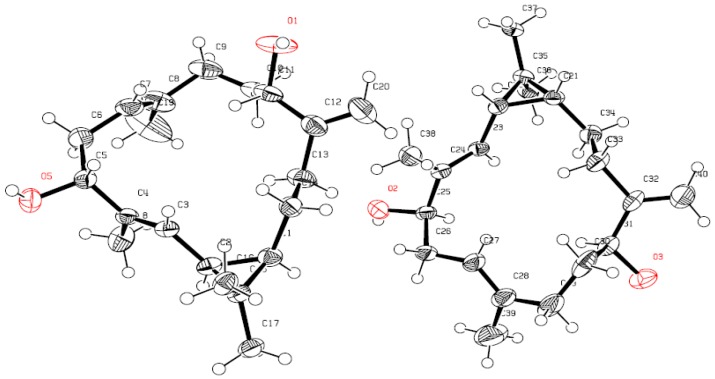
Figure 5.
ORTEP depictions of 2 with oxygens (O1–O5) labeled in red.
Compound 3 was obtained as a colorless oil with a positive optical rotation ( + 10.2 in MeOH). HR-ESI-FTMS analysis showed a molecular ion peak at m/z 355.1516 [M + Na]+ (calcd. for C19H24O5Na: 355.1521), consistent with a molecular formula C19H24O5, indicating eight degrees of unsaturation. Spectroscopic data was similar to previously reported 4 by Saitman, et al. in 2011, except around C-5, raising the possibility that 3 was an epimer of 4. Spectral shift differences included a downfield shift of H-5 (δH 4.42 d, J = 10.3 Hz) and an upfield shift of H-4 (2.61 and 2.49); downfield shifts for C-5, C-7, and C-4 in comparison with 4 were also observed [17,18,19]. The relative configuration of the four chiral centers at C-1, C-5, C-8, and C-10 were also determined by 13C signal comparisons as well as observed NOESY correlations (Figure 4). Assignment of H-1 to a β-orientation was based on Alcyonacea biogenesis of cembrane-type compounds [5,19]. In the NOESY spectrum, H-1 correlates with H-14β (δH 1.75 m) and one proton of C-2 methylene (δH 2.24, H-2β). NOESY correlations between H-5 (δH 4.42, d) with H-11α at (δH 7.20, s), H-4α at (δH 2.57, m), and H-10α at (δH 5.15 s) confirmed an α-orientation for H-5, indicating that epimerization occurs at C-5. Therefore, the norcembranoid was assigned to be the epimer of scabrolide F (sinularcasbane O).
In addition, previously reported metabolites scabrolide F (4) [18] and ineleganolide (5) [20] were identified by NMR and MS comparisons with literature values.
3. Materials and Methods
3.1. General Information
Specific rotation was measured with a Horiba SEPA-300 digital polarimeter (Kyoto, Japan, l = 5 cm) and IR spectra were collected on a Shimadzu FTIR-8100 spectrometer (Kyoto, Japan). For X-ray, a Bruker SMART-APEX II ULTRA (Billerica, MA, USA) was used. ESI-MS and HR-ESI-MS were carried out using a Thermo Fisher Scientific LTQ Orbitrap XL mass spectrometer (Waltham, MA, USA), and 1H (600 MHz) and 13C (150 MHz) NMR spectra were recorded on a JEOL JNM-ECA 600 spectrometer (Tokyo, Japan) with tetramethylsilane as an internal standard. Purification was run on a Shimadzu HPLC system equipped with a RID-10A refractive index detector and compound separation was performed on YMC-Pack ODS-A (Tokyo, Japan, 250 × 4.6 mm i.d.) and (250 × 20 mm i.d.) columns for analytical and preparative separation, respectively. Chromatography separation included normal-phase silica BW-200 (Fuji Silysia Chemical, Ltd., Kasugai, Japan, 150–350 mesh) and ODS reverse phase Chromatorex DM1020T (Fuji Silysia Chemical, Ltd., 100–200 mesh) columns as well as silica gel 60F254 (Merck, Darmstadt, Germany, 0.25 mm) and RP-18 WF254S (Merck, 0.25 mm) TLC plates with spots developed with heating of H2SO4-MeOH (1:9) sprayed plates.
3.2. Animal Material
Soft coral S. polydactyla was collected from the Egyptian Red Sea off the coast of Hurghada in March 2013. The soft coral was identified by Montaser A. Al-Hammady with a voucher specimen (03RS100) deposited in the National Institute of Oceanography and Fisheries, marine biological station, Hurghada, Egypt.
3.3. Extraction and Separation
Frozen soft coral (6.5 kg, total wet weight) was chopped into small pieces and extracted with methylene chloride/methanol (1:1) at room temperature (4 L × 5 times). The combined extracts were concentrated in vacuo to a brown gum. The dried material (243 g) was subjected to gravity chromatography in a silica gel column (6 cm × 120 cm) eluting with n-hexane (3000 mL) followed by a gradient of n-hexane-CH2Cl2 up to 100% CH2Cl2 and CH2Cl2–MeOH up to 50% MeOH (3000 mL each of the solvent mixture). The n-hexane/CH2Cl2 (1:2) fraction (2.2 g) eluted with n-hexane/EtOAc (6:1) was subjected to silica gel column separation. Fractions were obtained and combined into two main sub-fractions, A and B, according to a TLC profile. Sub-fraction A was re-purified by reversed-phase HPLC using MeOH/H2O (70%:30%) to afford 3 (11 mg), 4 (18 mg) and 5 (22 mg).
Sub-fraction B was re-purified by reversed-phase HPLC (Shimadzu HPLC system equipped with a RID-10A refractive index detector and compound separation was performed on YMC-Pack ODS-A (250 × 10 mm i.d.) column for separation) using MeOH/H2O (65%:35%) to afford 6 (10 mg). Sub-fraction C was re-purified by reversed-phase HPLC using MeOH/H2O (60%:30%) to afford 1 (11 mg) and 2 (18 mg).
Sinularcasbane M (1): colorless oil; = −9.6 (c 0.01, CHCl3); 1H-NMR and 13C-NMR data, see Table 1; HR-ESI-FTMS [M + Na]+ m/z 327.2293 (calc. 327.2300, C20H32O2Na); also see Figure S1.
Table 1.
1H- and 13C-NMR spectral data recorded in CDCl3, at 600 and 150 MHz, respectively.
| Position | 1 | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δC | |
| 1 | 0.69 (td, 10.9, 2.6) | 25.0 | 0.77 (ddd, 11.9, 8.9, 2.7) | 31.1 | 2.58 m | 40.8 | 38.8 |
| 2 | 1.26 (m) | 29.6 | 1.28 (dd, 10.1, 8.9) | 25.2 | 2.94 d (3.42), 2.96 d (2.04) | 50.2 | 50.2 |
| 3 | 5.19 (brd, 8.3) | 125.4 | 5.09 (dd, 10.1, 1.2) | 125.6 | 208.4 | 208.4 | |
| 4 | 137.9 | 136.8 | 2.47 m, 2.57 m | 44.0 | 44.0 | ||
| 5 | 4.39 ( dd, 10.0, 4.3) | 77.0 | 3.97 (dd, 10.7, 4.6) | 79.5 | 4.12 d (3.42), 4.14 (2.76) | 74.8 | 77.8 |
| 6 | 1.60 (ddd, 13.8, 11.1, 4.3) | 40.2 | 2.31 (ddd, 13.4, 10.7, 7.9) | 33.2 | 212.7 | 212.5 | |
| 1.94 (ddd, 13.8, 10.0, 2.2) | 2.38 (brdd, 13.4, 4.6) | ||||||
| 7 | 3.93 (brd, 11.1) | 71.2 | 4.86 (brdd, 7.9, 1.2) | 119.5 | 2.21 m, 2.39 m | 48.2 | 51.1 |
| 8 | 154.0 | 136.0 | 78.7 | 79.4 | |||
| 9 | 2.07 m | 33.4 | 1.98 (dd, 15.6, 7.6) | 34.3 | 2.11 m, 2.35 m | 43.1 | 41.7 |
| 2.19 m | 2.09 (ddd, 15.6, 13.1, 3.1) | ||||||
| 10 | 2.07 m | 24.8 | 1.52 (m) | 34.2 | 5.11 s | 78.3 | 79.0 |
| 2.19 m | 1.66 (m) | ||||||
| 11 | 5.02 (brd, 8.3) | 124.4 | 3.90 (dd, 7.6, 5.2) | 71.2 | 7.20 s | 150.7 | 151.6 |
| 12 | 136.9 | 154.5 | 131.2 | 131.1 | |||
| 13 | 1.80 m | 40.7 | 1.66 (m) | 34.1 | 1.92 m, 2.59 m | 20.8 | 20.1 |
| 2.19 m | 2.14 (ddd, 12.8, 12.8, 4.9) | ||||||
| 14 | 0.67 (td, 10.9, 4.0) | 31.5 | 1.13 (dddd, 13.4, 12.8, 11.9, 3.4) | 26.1 | 1.29 m, 1.75 m | 27.6 | 29.2 |
| 1.55 (dddd, 14.0, 13.4, 4.9, 2.7) | |||||||
| 15 | 20.1 | 20.1 | 145.8 | 145.4 | |||
| 16 | 1.00 s | 15.6 | 1.02 s | 15.5 | 4.67 s, 4.79 s | 113.1 | 113.0 |
| 17 | 1.06 s | 28.7 | 1.06 s | 29.1 | 1.64 s | 25.3 | 27.8 |
| 18 | 1.74 brs | 10.5 | 1.71 (d, 1.2) | 10.2 | 1.30 s | 18.1 | 18.4 |
| 19 | 4.89 brs | 109.8 | 1.63 (brs) | 17.1 | 174.0 | 174.4 | |
| 5.04 brs | |||||||
| 20 | 1.56 brs | 15.8 | 4.85 brs, | 108.6 | |||
| 5.02 brs | |||||||
Sinularcasbane N (2): colorless crystal; = −15.5 (c 0.01, CHCl3); 1H-NMR and 13C-NMR data, see Table 1; HR-ESI-FTMS [M + Na]+ m/z 327.2295 (calc. 327.2300, C20H32O2Na); also see Figure S1.
X-ray Crystallography Data
Single crystal X-ray analysis established the complete structure and absolute configuration of 2 and the crystal data are summarized as follows: C40H61O3, formula wt. 589.88, triclinic, space group, a = 9.437(4) Å, b = 9.499(4) Å, c = 10.543(4) Å, α = 98.763(6)°, β = 95.390(5)°, γ = 99.422(5)°, volume = 914.6(6) Å, are based upon the refinement of the XYZ-centroids of 5460 reflections above 20 σ(I) with 5.389° < 2θ < 41.63°. Data were corrected for absorption effects using the multi-scan method (SADABS). The ratio of minimum to maximum apparent transmission was 0.750. Dcacld = 1.071 g/cm3, crystal size 0.369 mm3 × 0.344 mm3 × 0.267 mm3. A total of 720 frames were collected, exposure time was 0.40 h and the frames were integrated with the Bruker SAINT Software package (V8.34A; Bruker AXS Inc., Madison, Wisconsin, USA, 2013) using a narrow-frame algorithm. The integration of the data using a triclinic unit cell yielded a total of 5460 reflections to a maximum θ angle of 27.85° (0.76 Å resolution), of which 4717 were independent (average redundancy 1.158, completeness = 95.8%, Rint = 2.69%, Rsig = 6.64%) and 3571 (75.70%) were greater than 2σ (F2). CCDC 1407158 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk.).
Sinularcasbane O (3): colorless oil; = +10.2 (c 0.01, CHCl3); 1H-NMR and 13C-NMR data, see Table 1; HR-ESI-FTMS [M + Na]+ m/z 355.1517 (calc. 355.1521 C19H24O5Na); also see Figure S1.
Scabrolide F (4): = −12.3 (c 0.01, CHCl3), HR-ESI-FTMS [M + Na]+ m/z 355.1516 (calc. 355.1521 C19H24O5Na).
Ineleganolide (5): colorless oil; = −35.0 (c 0.01, CHCl3), HR-ESI-FTMS [M + Na]+ m/z 353.1370 (calc. 353.1365 C19H22O5Na).
4. Conclusions
The methylene chloride/methanol (1:1) extract from the Red Sea coral S. polydactyla afforded three new metabolites, sinularcasbane M (1), sinularcasbane N (2) and sinularcasbane O (3). The structures were elucidated by spectroscopic analyses; the absolute configuration of 2 was confirmed by X-ray analysis.
Acknowledgments
This project was supported financially by the Science and Technology Development Fund (STDF), Egypt, Grant No. 1102.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/3/308/s1.
Author Contributions
Tarik A. Mohamed, Abdelsamed I. Elshamy contributed to extraction, isolation, identification and manuscript preparation. Montaser A. Al-Hammady contributed to soft coral collection and identification, Shinji Ohta and Paul W. Paré contributed to structure elucidation, guiding the experiments, analyses and manuscript writing. Mohamed-Elamir F. Hegazy is the project leader organizing and guiding the experiments, structure elucidation, and manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of compounds 2, 4, and 5 are available from the authors.
References
- 1.Anjaneyulu A.S.R., Venkateswarlu R. The chemical constituents of soft coral species of Sinularia genus: A review. J. Sci. Ind. Res. 1995;54:637–649. [Google Scholar]
- 2.Kamel H.N., Slattery M. Terpenoids of Sinularia: Chemistry and biomedical applications. Pharm Biol. 2005;43:253–269. doi: 10.1080/13880200590928852. [DOI] [Google Scholar]
- 3.Shaaban M., Shaaban K., Ghani M. Hurgadacin: A new steroid from Sinularia polydactyla. Steroids. 2013;78:866–873. doi: 10.1016/j.steroids.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Yin J., Zhao M., Ma M., Xu Y., Xiang Z., Cai Y., Dong J., Lei X., Huang K., Yan P. New casbane diterpenoids from a south China sea soft coral, Sinularia sp. Mar. Drugs. 2013;11:455–465. doi: 10.3390/md11020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H.Y., Yu S.J., Liu D., van Ofwegen L., Proksch P., Lin W.H. Sinularones A–I, new cyclopentenone and butenolide derivatives from a marine soft coral Sinularia sp. and their antifouling activity. Mar. Drugs. 2012;10:1331–1344. doi: 10.3390/md10061331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashita T., Nakao Y., Matsunaga S., Oikawa T., Imahara Y., Fusetani N. A new antiangiogenic C-24 oxylipin from the soft coral Sinularia numerosa. Bioorg. Med. Chem. 2009;17:2181–2184. doi: 10.1016/j.bmc.2008.10.083. [DOI] [PubMed] [Google Scholar]
- 7.Chai M.C., Wang S.K., Dai C.F., Duh C.Y. A cytotoxic lobane diterpene from the formosan soft coral Sinularia inelegans. J. Nat. Prod. 2000;63:843–844. doi: 10.1021/np990539e. [DOI] [PubMed] [Google Scholar]
- 8.Sheu J.H., Chang K.C., Duh C.Y. A cytotoxic 5α,8α-epidioxysterol from a soft coral Sinularia species. J. Nat. Prod. 2000;63:149–151. doi: 10.1021/np9903954. [DOI] [PubMed] [Google Scholar]
- 9.Chao C.H., Chou K.J., Huang C.Y., Wen Z.H., Hsu C.H., Wu Y.C., Dai C.F., Sheu J.H. Bioactive cembranoids from the soft coral Sinularia crassa. Mar. Drugs. 2011;9:1955–1968. doi: 10.3390/md9101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright A.D., Nielson J.L., Tapiolas D.M., Liptrot C.H., Motti C.A. A great barrier reef Sinularia sp. yields two new cytotoxic diterpenes. Mar. Drugs. 2012;10:1619–1630. doi: 10.3390/md10081619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards A.J., Head S.M. Key Environments-Red Sea. Pergamon Press; Oxford, UK: 1987. p. 440. [Google Scholar]
- 12.Hegazy M.E.F., Gamal Eldeen A.M., Shahat A.A., Abdel-Latif F.F., Mohamed T.A., Whittlesey B.R., Paré P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs. 2012;10:209–222. doi: 10.3390/md10010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegazy M.E.F., Mohamed T.A., Abdel-Latif F.F., Alsaid M.S., Shahat A.A., Paré P.W. Trochelioid A and B, new cembranoid diterpenes from the Red Sea soft coral Sarcophyton trocheliophorum. Phytochem. Lett. 2013;6:383–386. doi: 10.1016/j.phytol.2013.05.005. [DOI] [Google Scholar]
- 14.Elkhateeb A., El-Beih A.A., Gamal-Eldeen A.M., Alhammady M.A., Ohta S., Paré P.W., Hegazy M.E. New terpenes from the Egyptian soft coral Sarcophyton ehrenbergi. Mar. Drugs. 2014;12:1977–1986. doi: 10.3390/md12041977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Carbone M., Vitale R.M., Amodeo P., Castelluccio F., Sicilia G., Mollo E., Nappo M., Cimino G., Guo Y.W., et al. Rare casbane diterpenoids from the Hainan soft coral Sinularia depressa. J. Nat. Prod. 2010;73:133–138. doi: 10.1021/np900484k. [DOI] [PubMed] [Google Scholar]
- 16.Yang B., Huang J., Lin X., Liao S., Zhou X., Liu J., Wang J., Wang L., Liu Y. New casbane diterpenoids from the Hainan soft coral Sinularia species. Helv. Chim. Acta. 2015;98:834–841. doi: 10.1002/hlca.201400341. [DOI] [Google Scholar]
- 17.Saitman A., Rulliere P., Sullivan S.D.E., Theodorakis E.A. Total synthesis of norcembrenoilide A and scabrolide D. Org. Lett. 2011;13:5854–5857. doi: 10.1021/ol202476j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed A.F., Su J., Kuo Y., Sheu J. Scabrolides E–G, three new norditerpenoids from the soft coral Sinularia scabra. J. Nat. Prod. 2004;67:2079–2082. doi: 10.1021/np040112u. [DOI] [PubMed] [Google Scholar]
- 19.Chen W.T., Li Y., Guo Y.W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta. Pharm. Sin. B. 2012;2:227–237. doi: 10.1016/j.apsb.2012.04.004. [DOI] [Google Scholar]
- 20.Duh C.Y., Wang S.K., Chia M., Chiang M.Y. A novel cytotoxic norditerpenoid from the Formosan soft coral Sinularia inelegans. Tetrahedron Lett. 1999;40:6033–6035. doi: 10.1016/S0040-4039(99)01194-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.