Abstract
This paper is a review on the types of antagonists and the signaling mechanism pathways that have been used to determine the mechanisms of action employed for vasodilation by test compounds. Thus, we exhaustively reviewed and analyzed reports related to this topic published in PubMed between the years of 2010 till 2015. The aim of this paperis to suggest the most appropriate type of antagonists that correspond to receptors that would be involved during the mechanistic studies, as well as the latest signaling pathways trends that are being studied in order to determine the route(s) that atest compound employs for inducing vasodilation. The methods to perform the mechanism studies were included. Fundamentally, the affinity, specificity and selectivity of the antagonists to their receptors or enzymes were clearly elaborated as well as the solubility and reversibility. All the signaling pathways on the mechanisms of action involved in the vascular tone regulation have been well described in previous review articles. However, the most appropriate antagonists that should be utilized have never been suggested and elaborated before, hence the reason for this review.
Keywords: vasodilators, antagonists, signaling pathway, blood vessel, vascular tone
1. Introduction
Cardiovascular diseases are known to be the number one killer in the world, compared to other diseases such as disorders of the blood vessels and the heart. Hypertension is one of the main causes of cardiovascular diseases, and appropriately named as the silent killer due to it being an asymptomatic disease. In addition, it leads to a variety concomitant diseases, including stroke, heart diseases, kidney failure, cerebrovascular diseases, and more [1].Typically, hypertension has been categorized into four classes by the seventh report of Joint National Committee, which includes the normal and pre-hypertension stages, hypertension stage 1 and hypertension stage 2 [2]. Although there are many kinds of anti-hypertensive drugs present in the market nowadays, most have low effectiveness and undesired chronic side effects. Therefore, finding novel anti-hypertensive drugs is still a topic of huge interest to current researchers.
Hypertension is defined as having persistently high pressure exerted throughout the wall of blood vessels [3]. Blood vessels isolated from living organisms are commonly used for the in vitro studies on anti-hypertensive drugs researches [4]. Blood pressure has always been regulated in a narrow range to convey sufficient perfusion for tissues without causing any harm on the vascular system, especially the endothelium and vascular smooth muscle cells. Therefore, it is necessary to focus on understanding the signaling mechanism pathways involved in vascular tone regulation, including its way of signaling amplification by producing second messengers and the interaction between enzyme-linked, channel-linked, and G-protein coupled receptors. Plenty of reviews and research articles have discussed the probable signaling mechanism pathways involved in vascular tone regulation, providing great references for researchers, yet none of those discussed which of the signaling mechanisms pathway are significant, or suggest the most appropriate antagonists that should be utilized in respect to their corresponding receptors during studies of mechanistic pathways employed by the test compounds claimed to be exerting vasodilative effects. Therefore, the aim of this review is to provide a general view on the types of mechanistic pathways commonly employed by those researches that are related to this topic and the type of antagonists used. In addition, we aim to provide ideas on which antagonist is the most appropriate to use based on its specificity, selectivity, solubility, reversibility, and affinity to the receptors or enzymes. Finally, we aim to suggest the significance of signaling mechanism pathways to be employed.
This literature review focuses on the articles published during the latest five years (2010 to 2015) and abstracted in PubMed, strictly focusing on studies which meet at least two criteria: (a) the research must involve the use of blood vessels and (b) they must be studies of signaling pathway mechanism of action. Referring to these criteria, 257 articles were selected and reviewed in a comprehensive manner in order to meetthe aims of this review. We did not include in this review papers on reactive oxygen species and unrelated types of mechanistic studies.
2. Types of Blood Vessels
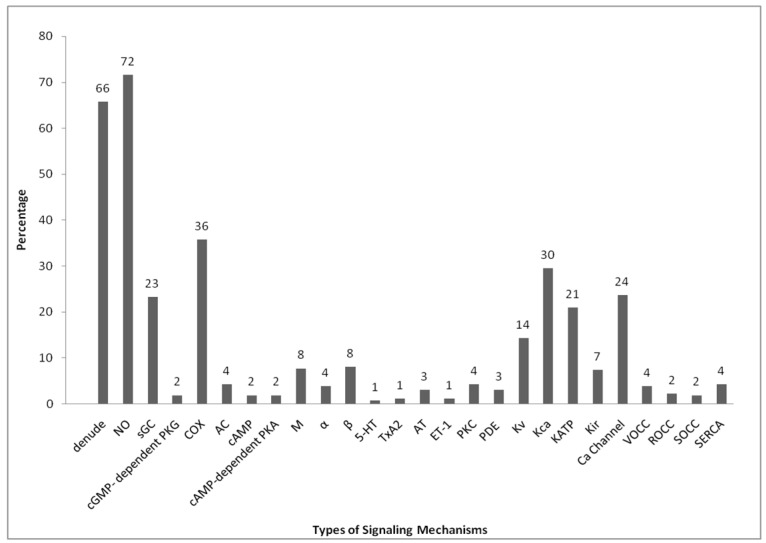
All studies involved a classical pharmacological approach using in vitro screening on isolated blood vessels, as shown in the Figure 1. A majority of the studies (67%) preferred the use of aorta rings for their in vitro studies on vasodilation, followed by mesenteric artery, coronary artery, pulmonary artery, renal artery, carotid artery, basilar artery, femoral artery, retinal arterioles, cerebral artery, and tail artery. Less than 1% of the studies were performed using saphenous vein, branchial artery, gonadal artery, internal mammary artery, caudal artery, prostatic small artery, afferent arterioles, gallbladder strips, ophthalmic artery, omental artery, bone resistance artery, splenic artery, iliac artery, umbilical artery, and gracilis arterioles.
Figure 1.
Percentage summary of signaling mechanism pathway studies conducted in research on blood vessel vasodilation published in PubMed from the year 2010 to 2015, as shown in Table 1. There were a total of 257 published research articles that performed research related to the vasodilation of aorta with studies on mechanisms of action within the specified period.
The rat aorta modeling design is the most commonly utilized method for studies on vascular function as well as studies on cell signaling. Using this method, the orientation variation of the smooth muscle cells can be reduced and the damage to the intima surface of the vessel can be minimized during isolation and hanging of the aorta inside the organ bath. In addition, the rat aorta model is a cost-effective, fast, and simple way for in vitro and in vivo vasodilation studies. Hence, it is known to be the “golden tool” in pharmacological researches [5].
3. Signaling Mechanisms Involved in Vasodilation Studies
The primary pathways involved in the vasodilation of blood vessels such as NO and COX are the most commonly used in the mechanism studies, since both of these pathways are well-studied EDRFs. They are subsequently followed by the potassium channels, which can be subdivided into Kca, KATP, Kv and Kir, channels. Calcium channels are essentially involved in the regulation of vascular tone, hence 24% of the researches in vasodilation studies were conducted on it. It is also because calcium channels can be divided into a few groups. Some of the researches that have been done were focused on those groups separately, such as VOCC, ROCC, SOCC, as well as the SERCA signaling pathways. The signaling pathway studies on enzyme-linked receptors such as sGC come after the calcium channels, while few researches had been done on AC. Other than those, a few other mechanisms such as PKG, cAMP, PKA, M, 5-HT, ET-1, TxA2, AT, PKC, PDE, and α- and β-adrenergic receptors have been included in these studies as well.
4. Endothelium-Derived Relaxing Factors (EDRFs)
In vascular tone regulation, both endothelium-derived relaxing factors (EDRFs) and endothelium-derived contracting factors (EDCFs) produce their effect via interaction between the endothelium and vascular smooth muscle cells. The most well-studied EDRFs are nitric oxide (NO), which is produced by endothelial nitric oxide synthases (eNOS), and prostacyclin (PGI2), produced by prostacyclin synthases within the endothelium [263,264,265,266,267].
4.1. Nitric Oxide (NO)
The highest percentage of the mechanism of actions summarized in Figure 1 belongs to the nitric oxide pathway at 72%, which are 184 out of 257 of the researches on this pathway. Nitric oxide is produced by the endothelial nitric oxide synthase (eNOS) in the endothelium from the breakdown of l-arginine. Therefore, to determine whether the relaxation induced by the extract is by this pathway, the specific inhibitor most commonly used is l-NG-NitroArginineMethyl Ester (l-NAME), followed by l-NG-NitroArginine (l-NNA). l-NNA is the first synthetic NOS inhibitor which acts competitively and selectively to eNOS [268]. However, l-NNA was claimed to bind with different isoforms. The binding of l-NNA to eNOS and nNOS is a time-dependent process with a relatively slow reversal [269,270]. l-NNA can show excellent stability in aqueous environment and has low toxicity, but due to its poor solubility at neutral pH, l-NAME is preferable, even though it is a weak NOS inhibitor [271,272]. The bioactivation of the l-NAME proceeds at a moderate rate in physiological buffers, but it is markedly accelerated in blood or the vascular endothelium [273]. Eventually, the percentage of relaxation caused by the extract will be reduced in the presence of eNOS inhibitor if the action of the active compound is via this pathway.
4.2. Prostacyclin (PGI2)
The second most frequently-studied mechanism of action is the prostacyclin (PGI2) signaling pathway (36%), which comprised 92 out of the 257 of the articles reviewed. PGI2 is produced by prostacyclin synthase from the intermediate prostaglandin H2, which is derived from AA and catalyzed by COX. Typically, COX can be classified into two types of isoforms. COX 1 is claimed to be responsible for the synthesis of prostaglandin and thromboxane, whereas the inducible COX 2 plays an important role in the synthesis of prostaglandin for the inflammatory cells as well as in the central nervous system [274,275]. From the literature, the studies on this pathway were commonly conducted using non-steroidal anti-inflammatory drugs (NSAIDs) as COX inhibitors. The most commonly used was indomethacin, followed by ibuprofen, meclofenamic acid, and diclofenac. Indomethacin is a potent inhibitor of both COX 1 and COX 2. However, there is an order of potency of the various inhibitors suggested, which is diclofenac > indomethacin > nimesulide ≈ meloxicam ≈ piroxicam [276]. Prostacyclin is one of the major relaxing factors derived from the endothelium. All the NSAIDs vary in their ability to inhibit both COX 1 and COX 2. Based on their mechanisms of inhibition, they can be classified into three broad categories. For instance, those that are able to bind reversibly and competitively to COX 1 and COX 2 are categorized in category 1. These include mefenamic acid, piroxicam, and ibuprofen. Those with rapid lower-affinity reversible binding, followed by the time-dependent with higher-affinity and slow reversible binding to both COX 1 and COX 2, form the second category, examples of which include diclofenac, flubiprofen, and indomethacin. Lastly, those with rapid reversible binding followed by covalent modification of both COXs, such as aspirin, are classified into category 3 [277,278]. Therefore, if the tested compound exhibits its vasodilator effect through the synthesis of prostacyclin, its percentage of relaxation will be significantly decreased in the presence of a COX inhibitor.
5. Enzyme-Linked NO pathway
Soluble Guanylyl Cyclase (sGC)
In vascular tone regulation, the soluble type of guanylyl cyclase (sGC) is strictly related to the NO that diffuses into the adjacent VSMCs. Some of the researchers assumed that it is one of the pathways of NO/cGMP and 23% (60 out of 257) of the research involved the study of this mechanism. Two types of inhibitors were utilized to study these mechanisms, which are methylene blue (MB) and 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ). MB was claimed to not be a true inhibitor of sGC. It can only act as a cGMP-lowering agent and partially prevents the nitrodilator-dependent activation of sGC by generating oxygen-free radicals. It has been described as a NO-release inhibitor [279] and does not enter the cells unless the membrane is permeabilized [280]. Therefore, MB is more appropriately used to determine the cGMP-dependent mechanism pathway since it is a non-selective inhibitor of sGC. However, the second type of inhibitor, ODQ, having an IC50 of around 20nM, was claimed to be a non-reversible but more selective inhibitor to the sGC enzyme [281]. ODQ can be solubilized by using DMSO and has been widely used to differentiate cGMP-mediated effects of NO from cGMP-independent effects [282,283]. ODQ apparently inhibits sGC through oxidation on the heme group of the enzyme. In this case, if the test compound exerts its relaxant effect through stimulation of the sGC activity, it will have a lower vasodilation effect on the isolated tissue in the presence of the ODQ inhibitor. Nonetheless, if the test compound’s relaxing effect is strictly dependent on the production of the cGMP, then the subsequent percentage of relaxation exerted on the isolated tissue will significantly decrease in the presence of methylene blue, or else it will remain unchanged in the presence of ODQ. A few studies (2% or 6 out of the 257 papers) included research on the protein kinase G (PKG) mechanism pathway. The most commonly used cGMP-dependent PKG inhibitor (cGKI) is Rp-8-Br-PET-cGMPs. It is a metabolically stable, competitive, reversible, and non-selective blocker since it was claimed to be able to block both PKG 1 and PKG 2 [284].
6. G-Protein-Coupled Receptors
G-protein-coupled receptor (GPCR) is a single polypeptide that has seven transmembrane-spanning α-helices. It will respond to ligands through the activation of the G-proteins, which are located on the intracellular surface of the cell membrane. In general, G-proteins can be categorized into α, β, and γ types. They are functionally switched on when bound to guanosine triphosphate (GTP) and will revert to resting state when they are bound to guanosine diphosphate (GDP) [285]. Once GTP is bound to the G-protein and activates it, the G-protein will be cleaved into Gα and Gβγ dimers. Typically, Gα-proteins can be classified into Gqα, Giα, and Gsα which are functionally responsible for different roles in signaling transduction of blood vessels. Gqα-protein tends to activate PLC-β for increasing the production of the second messengers IP3 and DAG, whereas Giα is functionally opposed to Gqα. Nonetheless, Gsα-protein tends to activate adenylyl cyclase (AC) to increase the production of the cAMP second messenger, which is involved in the vasodilation pathway. In the endothelium, there are a few Gqα-protein-coupled receptors such as angiotensin receptor (AT2) [286,287,288], serotonin (5-HT1D) receptor [289,290,291,292], bradykinin receptor (B2) [293,294], muscarinic receptor (M3), and endothelin receptor (ETBR) [295,296,297,298,299]. Whereas in the VSMC, the α1-adrenergic receptor [288,292], muscarinic receptor (M3) [300,301,302], angiotensin receptor (AT1) [286,303,304,305,306], endothelin receptor (ETAR & ETBR) [292,295,296,297,298,299,307], serotonin receptor (5-HT2) [289,290,292,308], and thromboxane receptor (TxA2) [288,292,309], are Gqα-protein-coupled receptors and tend to produce a direct effect in the increase of intracellular concentration of calcium by passing through the PLC-dependent pathway.
6.1. β-Adrenergic Receptors
Activation of the Gsα-protein-coupled receptors, such as the β2-adrenergic receptor and the IP receptor, will stimulate the activity of adenylyl cyclase (AC) in the VSMCs. These Gsα-PCRs have a less dominant presence in the VSMCs compared to Gqα-PCRs, hence less research were conducted on these mechanisms. Of the studies, only 4% (11 out of 257) were on the AC pathway, 2% (5 out of 257) on the cAMP, and 2% (5 out of 257) on the cAMP-dependent protein kinase A (PKA) pathway. Due to the PKA’s tetrameric composition in both catalytic and regulatory subunits, the inhibition of PKA can be achieved by blocking the ATP binding site or by inhibiting the analogue cAMP [310,311]. Generally, SQ22536 is more commonly utilized in vitro as an AC inhibitor, which is claimed to be more potent than MDL12330A. SQ22536 is specific to cAMP signaling and does not block the actions of other compounds that are not related to cAMP signaling [312]. SQ 22536 is cell-permeable and soluble in water. Other than that, Rp-cAMPs and H-89 have been used to study the cAMP and cAMP-dependent PKA mechanisms of action, respectively. Rp-cAMPs is a cell-permeable, reversible, competitive, and selective inhibitor for PKA. It is also resistant to the degradation of PDE, which makes it as an excellent tool to study these mechanisms [313]. H-89 is claimed to be a potent, cell-permeable, and reversible inhibitor of PKA, but it has been claimed that H-89 will exhibit unwanted effects such as influencing the ion currents and contraction of the smooth muscle [314] and activation of the PKG pathway [315]. Due to the unclear metabolic fate of H-89 and another inhibitor KT-5720, Rp-cAMPs is generally recommended for studies on this mechanism. In this case, if the test compound has employed this pathway for the regulation of the vascular tone, the expected outcome of the vasodilation effect would be decreased in the presence of this inhibitor. In regard to this pathway, the β2-adrenergic receptor, which is located on the VSMCs, actually employs this pathway for vasodilation as well. Only 8% (21 out of 257 studies) have focues onthis pathway. Even though its effect in the regulation of the vascular tone of blood vessels is not dominant, it is significant in myocytes. Propanolol is most widely used as the β-blocker in their studies. It is a non-selective blocker of the β-adrenergic receptor. Other than that, nadolol, atenolol, and pindolol have been used for these mechanistic studies as well. However, according to the classification of β-blockers, propanolol, nadolol, and pindolol are classified as non-selective β-blockers, whereas atenolol is more selective in cardio β1-blockers [316]. Propanolol is preferable for the screening of isolated tissue as compared to cardio-selective β-blockers. If the test compound involves this pathway, the resulting vasodilation effect will be decreased in the presence of β-blockers.
6.2. Muscarinic Receptors (M3)
There are five subgroups of muscarinic receptors, but only M3 receptors are present in the blood vessels. M3 receptors are Gqα-protein-coupled receptors, predominant in the endothelial cells in which they mediate vasodilative effect through stimulating the cascade of signaling pathways within the endothelium. There are M3 receptor selective antagonists present in the market, such as N-methylatropine and tiotropium, but evidence proves that M1 receptors are actually present in the endothelium [317]. However, that information still remains insufficient. Therefore, the non-selective muscarinic receptor atropine has been widely used to study this pathway, comprising approximately 8% (20 out of 257) of the studies. Atropine is a well-known muscarinic subtype non-specific antagonist, which acts as a competitive inhibitor with acetylcholine at the postganglionic muscarinic sites [318,319]. When atropine is bound to the M3 receptor, the subsequent vasodilation exerted by the test compound would be decreased significantly.
7. Vasoconstriction-Dominated Receptors
In studies about the mechanism pathways employed by test compounds causing vasodilation on isolated tissue, the mechanism of actions predominantly responsible for causing vasodilation should be well-determined This is especially true for eNOS, PGI2, sGC pathways, as well as their messengers produced. During these studies, researchers usually prioritize on enzymes and receptors which predominantly cause vasodilation upon their activation, such as eNOS, PGI2, AC, sGC, muscarinic receptors, and β-adrenoreceptors. This is because the vasodilation effect of the test substances should be dominating the effect of the vasoconstriction if the test substances in fact have a mild agonist effect on the vasoconstrictor-predominant receptors, such as serotonin receptors, ETAR, ETBR, AT-1, PKC, α1-adrenoreceptors, and TXA2 receptors. For instance, if the test substances have employed these signaling mechanism pathways, eventually they will result in vasoconstriction. Nonetheless, the test substances could act as blockers of these vasoconstriction-predominated receptors, which will cause more vasodilation. However, many of these mechanistic pathways might be included and need to add their effects to exhibit a major vasodilation effect. Therefore, most researchers did not conduct this type of mechanistic pathway studies since most of the results of each separate signaling mechanism study could be insignificant due to their minor contribution on the overall effect. From the articles, to determine the mechanism of action of the test compound, the percentage of relaxation in the experimental group where the antagonists have been applied before pre-contraction must be compared with the control group. However, from the data tabulation in Table 1, few studies have been conducted on the mechanism of action of vasoconstriction-dominated receptors. Those receptors, such as α1-adrenergic receptor (4%), serotonin receptor (1%), thromboxane receptor (1%), angiotensin 2 receptor (3%), and endothelin receptor (1%), are functionally dominant in the VSMCs. All these receptors are Gqα-protein-coupled receptors and tend to act in receptor-operated pathways to increase the calcium concentration in the cytosol when stimulated by their selective agonists. A selective blocker for α1-adrenergic receptor, prazosin, was the most commonly used test compound. There are two subtypes of endothelin receptors present in the VSMCs, BQ-123 and BQ-788, which have been used as selective blockers for ETAR and ETBR respectively. Besides, the angiotensin 2 type 1 receptor (AT-1) has been widely studied by using its specific antagonists, such as losartan and valsartan, whereas, the selective inhibitors for thromboxane receptor (TxA2) and serotonin (5-HT2) the compounds used were ridogrel and katanserin, respectively. Other than those, the protein kinase C (PKC) and PDE enzymatic signaling pathways have also been studied using their respective inhibitors. For instance, the selective inhibitors of PKC commonly used are GF 109203X, staurosporine, Go 6983, chelerythrine, and highly-selective cell-permeable BIM. PDE present in the VSCMCs are commonly classified as PDE 5 and PDE 3. About 3% of the papers involved studies on this pathway. Examples of selective inhibitors of PDE 5 are sildenafil, dipyridamole, zaprinast, and T-1032, while milrinone is a selective inhibitor for PDE 3. However, the non-selective PDE inhibitor papaverine has been utilized as well in their studies.
Table 1.
Data tabulation of the signaling mechanism pathways of vasodilation conducted in researches that were published in PubMed from the year 2010–2015.
| TOV | D | NO | GC | PKG | COX | AC | cAMP | PKA | M | α | β | Sr | T | AT | ET | PKC | PDE | Kv | Kca | KATP | Kir | Ca | V | R | S | SE | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | + | + | + | - | + | - | - | - | + | + | + | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | [6] |
| MA | + | + | + | - | + | + | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | + | - | - | - | - | [7] |
| A | + | + | + | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | [8] |
| A & SA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [9] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [10] |
| A | + | + | + | - | + | - | - | - | + | - | + | + | - | - | - | + | - | + | + | + | + | + | - | - | - | - | [11] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [12] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [13] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | [14] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [15] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | + | - | - | - | - | [16] |
| A | + | + | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [17] |
| MA | + | + | + | - | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [18] |
| A | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | [19] | ||
| A | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [20] |
| A & MA | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [21] |
| A | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | [22] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | [23] |
| RAS | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | [24] |
| A | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [25] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [26] |
| A | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | [27] |
| RAS | - | + | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | [28] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | + | - | - | - | - | [29] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | - | - | - | - | - | [30] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [31] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | - | - | - | - | - | [32] |
| A | + | + | + | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | + | + | - | + | - | - | + | + | [33] |
| A | + | + | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | + | + | + | + | - | - | - | - | [34] |
| CA | + | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | + | - | - | - | - | [35] |
| A | + | + | + | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [36] |
| A | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [37] |
| GA, BLA & A | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [38] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [39] |
| CA | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [40] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | + | - | - | - | [41] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | [42] |
| MA | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [43] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [44] |
| A | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | - | + | [45] |
| A | - | + | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [46] |
| A | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | [47] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [48] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | [49] |
| MA | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [50] |
| A | + | + | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [51] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [52] |
| A | + | - | - | - | - | - | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | [53] |
| IMA | - | + | + | - | + | - | - | - | + | - | - | - | - | - | + | - | - | + | + | + | + | - | - | - | - | - | [54] |
| A | + | + | - | - | - | - | - | - | - | + | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | [55] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | [56] |
| IMA | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | + | - | - | - | + | [57] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | + | + | - | - | - | - | [58] |
| A | + | + | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [59] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [60] |
| A | + | + | + | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | [61] |
| CBA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [62] |
| A | + | + | + | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [63] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [64] |
| A | + | + | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | [65] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [66] |
| MA | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | [67] |
| A | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | + | - | - | - | - | [68] |
| A | + | + | + | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | + | + | + | + | - | - | - | - | [69] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | [70] |
| CA | - | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [71] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [72] |
| A | + | + | - | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | [73] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [74] |
| A | + | + | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [75] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [76] |
| RA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | [77] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [78] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | [79] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [80] |
| A | - | + | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [81] |
| A | + | + | + | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | [82] |
| MA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | [83] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | [84] |
| A | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | - | - | - | - | [85] |
| A | + | - | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | [86] |
| A | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [87] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [88] |
| A | + | + | + | - | + | - | - | + | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [89] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [90] |
| A | + | + | + | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [91] |
| A | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [92] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [93] |
| A | + | + | + | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | + | + | - | + | - | - | + | + | [94] |
| A | + | + | + | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | + | + | - | + | - | - | + | + | [95] |
| CRA | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | + | - | - | + | + | [96] |
| A | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [97] |
| A | + | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | + | [98] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | + | - | - | - | - | [99] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [100] |
| A | - | + | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [101] |
| BA | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | + | - | - | - | - | [102] |
| A | + | + | + | - | + | - | - | - | + | - | + | - | - | - | - | - | - | + | + | + | - | + | + | + | - | - | [103] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [104] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [105] |
| A | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | [106] |
| A | + | + | + | - | + | - | - | - | - | - | + | - | - | - | - | + | - | + | + | + | - | + | - | - | - | - | [107] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | + | + | + | - | + | [108] |
| A | + | + | + | + | + | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [109] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | [110] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [111] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [112] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [113] |
| CA | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [114] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [115] |
| RA & A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [116] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [117] |
| RAS | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | + | + | - | - | - | [118] |
| A | + | + | - | - | + | - | - | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [119] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [120] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [121] |
| A & TA | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [122] |
| PA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [123] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | [124] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [125] |
| FA | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [126] |
| MA & A | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [127] |
| CA | + | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | [128] |
| CA & SPA | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | - | - | + | - | - | - | - | [129] |
| CRA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [130] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [131] |
| MA | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [132] |
| A | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [133] |
| A | + | + | + | - | + | - | - | - | + | - | + | - | - | - | - | - | - | - | + | + | - | + | - | - | + | + | [134] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [135] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [136] |
| PA | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [137] |
| PA | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [138] |
| BA | - | + | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [139] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [140] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [141] |
| A | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [142] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [143] |
| IA | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [144] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [145] |
| CA | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [146] |
| A | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [147] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [148] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [149] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [150] |
| RA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [151] |
| VA | - | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [152] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | [153] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | [154] |
| A | + | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | [155] |
| GCA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [156] |
| PA | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [157] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [158] |
| A | + | + | + | - | + | + | - | - | - | - | - | - | - | - | - | + | - | - | + | + | - | + | - | - | - | - | [159] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | + | - | - | - | - | - | [160] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [161] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [162] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [163] |
| MA | - | + | - | - | + | + | - | + | - | - | + | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | [164] |
| CA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [165] |
| A | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [166] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [167] |
| GCA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [168] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | [169] |
| A | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [170] |
| PA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [171] |
| A | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [172] |
| CA | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [173] |
| MA | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [174] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [175] |
| A | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [176] |
| CA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [177] |
| A | + | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [178] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + | + | + | - | - | [179] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + | + | + | - | - | [180] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [181] |
| FA | + | + | - | - | + | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | [182] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | [183] |
| CA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | [184] |
| A | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [185] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [186] |
| A | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | [187] |
| PA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [188] |
| A | + | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [189] |
| MA | + | + | + | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | [190] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [191] |
| A | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [192] |
| PA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | + | + | - | - | - | - | [193] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [194] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [195] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [196] |
| PA | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [197] |
| BA | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [198] |
| PA | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [199] |
| BA | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | [200] |
| PSA | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | + | - | - | - | - | [201] |
| A | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [202] |
| MA | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [203] |
| CA & FA | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [204] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + | + | + | - | - | [205] |
| RA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [206] |
| AA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [207] |
| A | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [208] |
| A | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [209] |
| TA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [210] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [211] |
| A | + | + | - | - | - | - | - | + | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | - | - | [212] | |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | + | - | - | - | - | [213] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | - | - | + | + | + | - | - | [214] |
| PA | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | [215] |
| RAS | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | [216] |
| MA | - | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [217] |
| CA | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [218] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | + | [219] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [220] |
| A | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [221] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [222] |
| MA | - | + | + | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | + | - | - | - | [223] |
| CRA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | [224] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [225] |
| A, CA, MA & RA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [226] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [227] |
| A & FA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [228] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [229] |
| A | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [230] |
| RA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | - | [231] |
| MA | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [232] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [233] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [234] |
| MA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [235] |
| RSA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [236] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [237] |
| A | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [238] |
| A | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [239] |
| MA | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [240] |
| GS | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | [241] |
| OPA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [242] |
| A | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [243] |
| CRA | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [244] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [245] |
| MA | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [246] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [247] |
| CA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | [248] |
| MA | - | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [249] |
| CBA, BA & MA | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | [250] |
| CA | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [251] |
| A | + | + | + | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | [252] |
| A | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [253] |
| A & MA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [254] |
| OPA | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [255] |
| MA & OMA | + | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | [256] |
| A | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [257] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | [258] |
| A | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [259] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [260] |
| A | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [261] |
| A | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | [262] |
Abbreviations: +, performed; -, not performed; A, aorta; AA, afferent arterioles; AT, angiotensin II; BA, basilar artery; BLA, bronchial artery; BRA, bone resistance artery; CA, coronary artery; CBA, cerebral artery; CDA, caudal artery; CRA, carotid artery; D, denuded; ET, endothelin; FA, femoral artery; GA, gonadal artery; GCA, gracilis arterioles; GS, gallbladder strips; IA, iliac artery; IMA, internal mammary artery; MA, mesenteric artery; OMA, omental artery; OPA, ophthalmic artery; PA, pulmonary artery; PSA, prostatic small artery; R, receptor-operated calcium channels; RA, renal artery; RAS, retinal arterioles; RSA, resistance artery; S, store-operated calcium channels; SA, saphenous artery; SE, Sacro/endoplasmic reticulum Ca2+-ATPase; SPA, splenic artery; Sr, serotonin; SV, saphenous vein; T, thromboxane A2; TA, tail artery; TOV, type of vessel; UA, umbilical artery; V, voltage-operated calcium channels; VA, vertebrobasilar artery.
8. Channel-Linked Receptors
These receptors are also known as ligand-gated channels, whereby the channels respond by binding with chemical messengers and react by allowing the ions, such as calcium, potassium, sodium, chloride, or magnesium, to enter or leave the cell. Typically, the reaction of these receptors is regulated by the membrane potential.
8.1. Potassium Channels
8.1.1. Calcium-Dependent Potassium Channels (Kca)
According to Table 1, thisis the 3rd most abundantly studied topic (30%, 76 out of 257 papers) in the reviewed research. Kca channels consist of small-conductance (SKca), intermediate-conductance (IKca), and big-conductance calcium-activated potassium channels (BKca). SKca and IKca channels are more abundantly expressed in the endothelium, but expressed poorly in the VSMCs, hence it has been claimed they are able to contribute to the EDHF signals and react in the adjacent VSMCs [320,321,322,323,324,325,326], whereas the BKca channels are expressed in virtually all VSMCs. According to the analysis, the majority of the studies on this pathway have been conducted using the non-selective Kca channel blocker, tetraethylammonium ion (TEA). Even though TEA can completely abolish the delayed potassium channels current without affecting the transient sodium current [327], it is a non-selective Kca channel blocker. Therefore, to make the Kca channel signaling pathway clearer, SKca, IKca, and BKca channel blockers should be used to examine the Kca channel pathway mechanisms. Due to this reason, some of the researches were done by using different selective channel blockers. Blockers such as apamin were used as the SKca channel blocker, TRAM-34 and clotrimazole as selective blockers for IKca, and iberiotoxin, UCL 1684, and paxilline as the selective blockers for BKca channels [323]. A few of the researches have used charybdotoxin as a non-selective blocker to determine both BKca and IKca channels. Specific determination of both IKca and SKca channels is necessary due to their contribution as EDHF within the endothelium. Besides, both of these channels are functionally associated with calcium and calmodulin binding [328], and are indirectly involved in the regulation of vascular tone through their participation in generating membrane potential. The activation of the BKca channels are indirectly mediated by many of the second messengers such as PKA, PKG, and the nitric oxide that diffuse across the VSMC membrane. BKca channels are voltage and calcium concentration-dependent potassium channels, hence indicating their essential roles in controlling the vascular tone excitability. The vasodilation effect will be decreased in the presence of the BKca inhibitor since there is a high depolarizing voltage created on the VSMC membrane that can induce more influx of calcium ions into the cytosol and cause muscle contraction.
8.1.2. ATP-Sensitive Potassium Channels (KATP)
The second most frequently used channel in vasodilation research was ATP-sensitive potassium channels (KATP), which were studied in 21% (54 out of 257) of the reports. This channel opens when the intracellular ATP concentration falls below 1 mmol/L [329,330]. Glibenclamide, a selective KATP channels blocker, is the only antagonist utilized by those researches to determine thismechanism of action [331]. A few endothelium-derived substances, such as calcitonin-gene related protein (CGRP) and hydrogen sulfide (H2S), are able to stimulate KATP channels directly as well as indirectly mediated by cAMP-dependent PKA. The opening of these channels can cause membrane hyperpolarization, therefore the vasodilation effect will be decreased when the KATP channel antagonists are present [330].
8.1.3. Voltage-Dependent Potassium Channels (Kv) and Inwardly Rectifier Potassium Channels (Kir)
There are two more potassium channels which are important based on their functional roles in the regulation of membrane potential, namely voltage-activated potassium channels (Kv) and inwardly rectifier potassium channels (Kir), which have been studied in 14% (37 out of 257) and 7% (19 out of 257) of the studies, respectively. They are less significant when compared to Kca channels and KATP channels because their functional roles are strictly regulated through the membrane potential. To determine the Kv channel pathway mechanisms, all the researchers used 4-aminopyridine (4-AP) as an antagonist and only one group used the Kv7 selective blocker to investigate this pathway. Kv channels consist of many different subgroups, therefore in order to inhibit these channels, a non-selective 4-AP blocker is preferable to using a selective inhibitor. It is an important component of the outward potassium conductance and is heavily regulated by kinases such as PKA, PKG and PKC, while sGC and NO tend to activate this channel directly [323]. These channels tend to provide the counterbalancing potassium efflux for the calcium influx through the voltage-operated calcium channel (VOCC) [332,333]. Therefore, if these channels were blocked, subsequent membrane depolarizing current will induce the calcium influx, resulting in more vasoconstriction. The Kir channels more readily induce the inward movement of potassium current and fasten the membrane potential to the resting stage [323]. Barium chloride (BaCl2) is the Kir channel blocker that has been most used so far according to the tabulated data, and will cause the decrease in relaxation response if the test compound employed this pathway. Barium chloride can selectively block Kir channels [334].
8.2. Calcium Channels
8.2.1. Voltage-Operated Calcium Channels (VOCC)
Generally, there are three types of commonly-used selective L-type calcium channel blockers from the data obtained, which are the dihydropyridine class of nifedipine, the benzothiazepine class of diltiazem, and the phenylalkylamineclass of verapamil. These three classes of calcium channel blockers have different pharmacological effects due to the fact that they bind to different sites of the calcium channel. Diltiazem and verapamil have overlapping binding sites, whereas nifedipine binds to a distinct site. Therefore, the selectivity to the calcium channel blocker is arranged in ascending order as such nifedipine>diltiazem>verapamil, with the experiments executed using peripheral arterioles or coronary arteries [335]. However, verapamil is still the most widely-used calcium channel blocker in the studies. The dihydropyridines calcium channels blockers, such as nifedipine and nicardipine, have a higher vascular selectivity [336] and are the most smooth muscle selective class of the calcium channel blockers. From Table 1, 4% (10 out of 257) of the studies were conducted regarding this calcium channel blocker. Two types of methods were implemented in the researches to determine the VOCC mechanism pathway. One of the methods is that isolated tissue was incubated with the calcium channel antagonist before pre-contraction, and subsequently the cumulative concentration of the test compound was added. In this case, if the test compound has the potential to act as a VOCC opener in their mechanism of action in controlling the vascular tone in the control set of experiment, then the resulting vasodilation should be increased in the second set of the experiments where the VOCC antagonist was applied. By using this method, the inference of whether the test compound has employed this pathway as its mechanism of action can be made, but is not practically used because the action potential would abolish the significance of the result achieved. Therefore, the second method was the choice of the majority of researchers, as it is more suitable to determine whether their test compound can be a potential blocker for the VOCC. Isolated tissue is incubated and washed with calcium-free Krebs-Henseleit solution, which contains ethylenediamine tetraacetic acid (EDTA) or ethylene glycol tetraacetic acid (EGTA) with high potassium content (~50 mM). Both of them are chelating agents and appear as white crystalline powders, but EGTA is preferable because of its significantly higher affinity to the divalent calcium ions, while EDTA is preferable for divalent magnesium ions. The solution used to wash the isolated tissue should not contain any EGTA or EDTA. The tissue was then incubated with certain doses of the test compound for at least 10 min before adding cumulative doses of calcium chloride. The experimental group was compared to the control group (including the positive control) to determine the degree of significance. By using this method, if the contraction induced by the cumulative doses of calcium chloride is significantly decreased, this indicates that the test compound has potential to act as the antagonist of the VOCC.
8.2.2. Store-Operated Calcium Channels (SOCC)
Regarding the intracellular release of calcium from the sacroplasmic reticulum stores, gandolinium (Gd3+) and 2-aminoethoxydiphenyl borate (2-APB) are most widely been used inhibitors to study this pathway. Around 2% of the researcher studied this pathway. 2-APB is an IP3R inhibitor and is able to inhibit the release of the intracellular stores of calcium from SR. It was claimed to be able to completely inhibit the release of calcium at high concentrations [337,338] and is also a reliable blocker of the SOCC, but an inconsistent inhibitor of IP3-induced calcium release [339]. Other than that, the Gd3+ is usually used together with the 2-APB as it is a selective blocker of the SOCC [340,341]. However, a few of the researchers were using non-competitive, selective, and cell-permeable SERCA blockers such as thapsigargin [342] to aid the calcium entry blocking effect from the SOCC into the SR during their studies on this mechanism of action. Like VOCC, two methods can be used to test this SOCC mechanism of action. The isolated tissue is pre-treated with the normal Krebs-Henseleit solution containing thapsigargin, Gd3+, as well as nifedipine to eliminate the VOCC effect for at least 10 min before the pre-contraction. Subsequently, cumulative doses of the test compound were added. If the test compound utilizes this pathway in its regulation of vascular tone, it will exhibit more relaxation than in the control group because the test compound can no longer induce the entry of calcium though the SOCC. However, another method is used to determine whether the calcium released from the sites of intracellular calcium store contributes to the auto-regulation of vascular tone. In this case, the isolated tissue was pre-treated with calcium-free Krebs-Henseleit solution containing the test compound for at least 20 min before the addition of phenylephrine. If there is a significant change in the percentage of contraction induced by PE, this indicates that the test compound plays a role in controlling the intracellular release of calcium into the cytosol.
9. List of Antagonists and Its Receptor
All the antagonists used in their respective signaling mechanism pathway studies as tabulated in Table 1 were accordingly recategorized in Table 2 to facilitate future researchers in related pharmacological research fields.
Table 2.
General list of antagonists used in studies of signaling mechanism pathways according to their selectivity.
| Mechanism Pathways | Antagonists | Ref. | |
|---|---|---|---|
| Selective | Non-Selective | ||
| Endothelial nitric oxide synthase (eNOS) | l-NG-NitroArginine-Methyl Ester, l-NG-NitroArginine | [268,269,270,271,272,273] | |
| Cyclooxygenase (COX) | Ibuprofen, mefenamic acid, piroxicam, diclofenac, flubiprofen, indomethacin, aspirin | [276,277,278] | |
| Soluble guanylyl cycles (sGC) | 1H-[1,2,4] oxadiazolo [4,3-a]-quinoxalin-1-one | Methylene blue | [279,281,282,283] |
| Protein kinase G (PKG) | KT 5823 | Rp-8-Br-PET-cGMPs | [284] |
| Adenylyl cyclase (AC) | SQ22536, MDL12330A, 2′5′-dideoxyadenosine | [312] | |
| Protein kinase A (PKA) | Rp-cAMPs, KT 5720, H-89 | [313,314,315] | |
| β-adrenergic receptor | Atenolol | Propanolol, nadolol, pindolol | [316] |
| α-adrenergic receptor | Prazosin (α1), RX 821002 (α2) | [288,292] | |
| Endothelin receptor | BQ 123 (ETAR), BQ 788 (ETBR) | Bosentan | [295,296,297,298,299] |
| Angiotensin II receptor (AT1) | Candesartan cilexetil (ARB & AT1), losartan (AT1), valsartan (ARB & AT1) | [286,287,288,303,304,305,306] | |
| Muscarinic receptor | N-methylatropine, tiotropium | Atropine | [300,301,302,318,319] |
| Thromboxane (TxA2) | Ridogrel, ozagrel, furegrelate | [288,292,309] | |
| Serotonin receptor (Sr) | Katanserin (5-HT2), WAY 100635 (5-HT1A) | [289,290,291,292] | |
| Protein kinase C (PKC) | GF 109203x, staurosporine, Go 6983, chelerythrine, BIM | [286,287,288] | |
| Phosphodiesterase (PDE) | Slidenafil (PDE 5), dipyridamole (PDE 5), zaprinast (PDE 5), T 1032 (PDE 5), rolipram (PDE 4), milrinone (PDE 3) | Papaverine | [152] |
| Calcium-activated potassium channel (Kca) | Iberiotoxin (BKca), UCL 1684 (BKca), paxilline (BKca), apamin (SKca), TRAM-34 (IKca), clotrimazole (IKca) | Charybdotoxin (BKca & IKca), TEA | [323,327,328] |
| ATP-sensitive potassium channel (KATP) | Glibenclamide | [330,331] | |
| Voltage-activated potassium channel (Kv) | XE 991 (KV 7) | 4-aminopyridine | [323,332,333] |
| Inwardly-rectifier potassium channel (Kir) | Barium chloride | [334] | |
| Voltage-operated calcium channel (VOCC) | Nifedipine, nicardipine, diltiazem | Verapamil | [335,336] |
| Store-operated calcium channel (SOCC) | Gandolinium | [340,341] | |
| Inositol triphosphate receptor (IP3R) | 2-Aminoethoxydiphenyl borate | [337,338,339,340,341] | |
| SERCA | Thapsigargin, cyclopiazonic acid | [342] | |
Abbreviation: (), selective in receptor.
10. Conclusions
Understanding the microenvironment of blood vessel in regard to its regulation of vascular tone is essential for those who work in research to develop a new anti-hypertensive drug. Nonetheless, during the discovery of new drugs, researchers should be able to confirm and elaborate the most appropriate types of antagonists used according to their selectivity, specificity, affinity, reversibility and solubility to test on potential anti-hypertensive test compounds as well as the procedures used in order to obtain a clear and firm inference about the pathways that the test compound has employed for inducing the vasodilation effect. In this review paper, the essential signaling mechanism pathways that were most frequently demonstrated in anti-hypertensive research have been summarized, as well as the expected outcome of the studies of test compounds that involve a particular mechanism pathway. In the future, a general picture of the blood vessel microenvironment in vascular tone regulation, including signals of second messengers and the interaction among receptors and enzymes, should be used as a whole to provide a general view for the researchers who will work on this topic.
Acknowledgments
We extend the sincerest apologiesto any authorswhose work was not cited in this review. We would also like to thank those who have contributed their ideas in this topic. This review article was not supported by any grant.
Author Contributions
Y.C.L. prepared the manuscript; C.S.T. searched and reviewed published articles in PubMed; Y.S.C. recorded and analyzed data; M.A., M.Z.A. and M.F.Y. planned and coordinated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yang Y., Zhang Z., Li S., Ye X., Li X., He K. Synergy effects of herb extracts: pharmacokinetics and pharmacodynamic basis. Fitoterapia. 2014;92:133–147. doi: 10.1016/j.fitote.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Lenfant C., Chobanian A.V., Jones D.W., Roccella E.J. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 3.Whalen K. Lippincott Illustrated Reviews: Pharmacology. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2014. pp. 225–240. [Google Scholar]
- 4.Yildiz O., Gul H., Seyrek M. Pharmacology of Arterial Grafts for Coronary Artery Bypass Surgery. Intech Open Access Publisher; Rijeka, Croatia: 2013. [Google Scholar]
- 5.Rameshrad M., Babaei H., Azarmi Y., Fouladia D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016;145:190–204. doi: 10.1016/j.lfs.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 6.Ameer O.Z., Salman I.M., Siddiqui M.J., Yam M.F., Sriramaneni R.N., Mohamed A.J., Sadikun A., Ismail Z., Shah A.M., Asmawi M.Z. Pharmacological mechanisms underlying the vascular activities of Loranthus ferrugineus Roxb. in rat thoracic aorta. J. Ethnopharmacol. 2010;127:19–25. doi: 10.1016/j.jep.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 7.Li R.W., Yang C., Shan L., Zhang Z., Wang Y., Kwan Y.W., Lee S.M., Hoi M.P., Chan S.W., Cheung A.C., et al. Relaxation effect of a novel Danshensu/tetramethylpyrazine derivative on rat mesenteric arteries. Eur. J. Pharmacol. 2015;761:153–160. doi: 10.1016/j.ejphar.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Tsao C.M., Chen S.J., Tsou M.Y., Wu C.C. Effect of propofol on vascular reactivity in thoracic aortas from rats with endotoxemia. J. Chin. Med. Assoc. 2012;75:262–268. doi: 10.1016/j.jcma.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Davis B., Rahman A., Arner A. AMP-activated kinase relaxes agonist induced contractions in the mouse aorta via effects on PKC signaling and inhibits NO-induced relaxation. Eur. J. Pharmacol. 2012;695:88–95. doi: 10.1016/j.ejphar.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Csanyi G., Gajda M., Franczyk-Zarow M., Kostogrys R., Gwozdz P., Mateuszuk L., Sternak M., Wojcik L., Zalewska T., Walski M., et al. Functional alterations in endothelial NO, PGI(2) and EDHF pathways in aorta in ApoE/LDLR-/- mice. Prostaglandins Other Lipid Mediat. 2012;98:107–115. doi: 10.1016/j.prostaglandins.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Kim E.Y., Lee Y.J., Rhyu M.R. Black cohosh (Cimicifuga racemosa) relaxes the isolated rat thoracic aorta through endothelium-dependent and -independent mechanisms. J. Ethnopharmacol. 2011;138:537–542. doi: 10.1016/j.jep.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Hernandez J.M., Ramirez-Lee M.A., Rosas-Hernandez H., Salazar-Garcia S., Maldonado-Ortega D.A., Gonzalez F.J., Gonzalez C. Single-walled carbon nanotubes (SWCNTs) induce vasodilation in isolated rat aortic rings. Toxicol. In Vitro. 2015;29:657–662. doi: 10.1016/j.tiv.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Capettini L.S., Cortes S.F., Lemos V.S. Relative contribution of eNOS and nNOS to endothelium-dependent vasodilation in the mouse aorta. Eur. J. Pharmacol. 2010;643:260–266. doi: 10.1016/j.ejphar.2010.06.066. [DOI] [PubMed] [Google Scholar]
- 14.Choi S., Kim H.I., Park S.H., Lee M.J., Jun J.Y., Kim H.L., Chung J.H., Yeum C.H. Endothelium-dependent vasodilation by ferulic acid in aorta from chronic renal hypertensive rats. Kidney Res. Clin. Pract. 2012;31:227–233. doi: 10.1016/j.krcp.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziberna L., Lunder M., Tramer F., Drevensek G., Passamonti S. The endothelial plasma membrane transporter bilitranslocase mediates rat aortic vasodilation induced by anthocyanins. Nutr. Metab. Cardiovasc. Dis. 2013;23:68–74. doi: 10.1016/j.numecd.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Koon C.M., Fong S., Wat E., Wang Y.P., Wing-Shing Cheung D., Bik-San Lau C., Leung P.C., Sun H.D., Zhao Q.S., Fung K.P. Mechanisms of the dilator action of the Erigerontis Herba on rat aorta. J. Ethnopharmacol. 2014;155:1561–1567. doi: 10.1016/j.jep.2014.07.053. [DOI] [PubMed] [Google Scholar]
- 17.Bertin R., Chen Z., Martinez-Vazquez M., Garcia-Argaez A., Froldi G. Vasodilation and radical-scavenging activity of imperatorin and selected coumarinic and flavonoid compounds from genus Casimiroa. Phytomedicine. 2014;21:586–594. doi: 10.1016/j.phymed.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey S.H., Liu L., Chappell M.C. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids. 2014;81:99–102. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shou Q., Pan Y., Xu X., Xu J., Wang D., Ling Y., Chen M. Salvianolic acid B possesses vasodilation potential through NO and its related signals in rabbit thoracic aortic rings. Eur. J. Pharmacol. 2012;697:81–87. doi: 10.1016/j.ejphar.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 20.Cordeiro B., Shinn C., Sellke F.W., Clements R.T. Rottlerin-induced BKCa channel activation impairs specific contractile responses and promotes vasodilation. Ann. Thorac. Surg. 2015;99:626–634. doi: 10.1016/j.athoracsur.2014.07.091. [DOI] [PubMed] [Google Scholar]
- 21.Yamawaki H., Tsubaki N., Mukohda M., Okada M., Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem. Biophys. Res. Commun. 2010;393:668–672. doi: 10.1016/j.bbrc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 22.Xu Z., Wang X., Dai Y., Kong L., Wang F., Xu H., Lu D., Song J., Hou Z. (+/−)-Praeruptorin A enantiomers exert distinct relaxant effects on isolated rat aorta rings dependent on endothelium and nitric oxide synthesis. Chem. Biol. Interact. 2010;186:239–246. doi: 10.1016/j.cbi.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Celotto A.C., Restini C.B., Capellini V.K., Bendhack L.M., Evora P.R. Acidosis induces relaxation mediated by nitric oxide and potassium channels in rat thoracic aorta. Eur. J. Pharmacol. 2011;656:88–93. doi: 10.1016/j.ejphar.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 24.Mori A., Suzuki S., Sakamoto K., Nakahara T., Ishii K. Vasodilation of retinal arterioles induced by activation of BKCa channels is attenuated in diabetic rats. Eur. J. Pharmacol. 2011;669:94–99. doi: 10.1016/j.ejphar.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Tsounapi P., Saito M., Kitatani K., Dimitriadis F., Ohmasa F., Shimizu S., Kinoshita Y., Takenaka A., Satoh K. Fasudil improves the endothelial dysfunction in the aorta of spontaneously hypertensive rats. Eur. J. Pharmacol. 2012;691:182–189. doi: 10.1016/j.ejphar.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Silva B.R., Pernomian L., Grando M.D., Amaral J.H., Tanus-Santos J.E., Bendhack L.M. Hydrogen peroxide modulates phenylephrine-induced contractile response in renal hypertensive rat aorta. Eur. J. Pharmacol. 2013;721:193–200. doi: 10.1016/j.ejphar.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Silva B.R., Pernomian L., Grando M.D., Bendhack L.M. Phenylephrine activates eNOS Ser 1177 phosphorylation and nitric oxide signaling in renal hypertensive rat aorta. Eur. J. Pharmacol. 2014;738:192–199. doi: 10.1016/j.ejphar.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Nakabayashi S., Nagaoka T., Tani T., Sogawa K., Hein T.W., Kuo L., Yoshida A. Retinal arteriolar responses to acute severe elevation in systemic blood pressure in cats: Role of endothelium-derived factors. Exp. Eye Res. 2012;103:63–70. doi: 10.1016/j.exer.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Guven G., Seyrek M., Vural I.M., Cehreli Z.C., Yildiz O. Vasodilatory effect of hydroxyethyl methacrylate and triethylene glycol dimethacrylate in rat aorta through calcium antagonistic action. J. Endod. 2011;37:353–357. doi: 10.1016/j.joen.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 30.Li H., Hong da H., Son Y.K., Na S.H., Jung W.K., Bae Y.M., Seo E.Y., Kim S.J., Choi I.W., Park W.S. Cilostazol induces vasodilation through the activation of Ca2+-activated K+ channels in aortic smooth muscle. Vascul. Pharmacol. 2015;70:15–22. doi: 10.1016/j.vph.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Kamkaew N., Scholfield C.N., Ingkaninan K., Maneesai P., Parkington H.C., Tare M., Chootip K. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J. Ethnopharmacol. 2011;137:790–795. doi: 10.1016/j.jep.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 32.Ng C.F., Koon C.M., Cheung D.W., Lam M.Y., Leung P.C., Lau C.B., Fung K.P. The anti-hypertensive effect of Danshen (Salvia miltiorrhiza) and Gegen (Pueraria lobata) formula in rats and its underlying mechanisms of vasorelaxation. J. Ethnopharmacol. 2011;137:1366–1372. doi: 10.1016/j.jep.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Jin S.N., Wen J.F., Wang T.T., Kang D.G., Lee H.S., Cho K.W. Vasodilatory effects of ethanol extract of Radix Paeoniae Rubra and its mechanism of action in the rat aorta. J. Ethnopharmacol. 2012;142:188–193. doi: 10.1016/j.jep.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 34.Qu Z., Zhang J., Gao W., Chen H., Guo H., Wang T., Li H., Liu C. Vasorelaxant effects of Cerebralcare Granule® are mediated by NO/cGMP pathway, potassium channel opening and calcium channel blockade in isolated rat thoracic aorta. J. Ethnopharmacol. 2014;155:572–579. doi: 10.1016/j.jep.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 35.Hu F., Koon C.M., Chan J.Y., Lau K.M., Kwan Y.W., Fung K.P. Involvements of calcium channel and potassium channel in Danshen and Gegen decoction induced vasodilation in porcine coronary LAD artery. Phytomedicine. 2012;19:1051–1058. doi: 10.1016/j.phymed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Chen H., Li S., Wang P., Yan S., Hu L., Pan X., Yang C., Leung G.P. Endothelium-dependent and -independent relaxation of rat aorta induced by extract of Schizophyllum commune. Phytomedicine. 2014;21:1230–1236. doi: 10.1016/j.phymed.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Khanna V., Jain M., Barthwal M.K., Kalita D., Boruah J.J., Das S.P., Islam N.S., Ramasarma T., Dikshit M. Vasomodulatory effect of novel peroxovanadate compounds on rat aorta: Role of rho kinase and nitric oxide/cGMP pathway. Pharmacol. Res. 2011;64:274–282. doi: 10.1016/j.phrs.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Cameron M.S., Nobata S., Takei Y., Donald J.A. Vasodilatory effects of homologous adrenomedullin 2 and adrenomedullin 5 on isolated blood vessels of two species of eel. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015;179:157–163. doi: 10.1016/j.cbpa.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 39.Tom E.N., Girard C., Dimo T., Mbafor J.T., Berthelot A., Demougeot C. Vasorelaxant effects of extracts of the stem bark of Terminalia superba Engler & Diels (Combretaceae) J. Ethnopharmacol. 2010;127:335–340. doi: 10.1016/j.jep.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 40.Neubauer R., Wolkart G., Opelt M., Schwarzenegger C., Hofinger M., Neubauer A., Kollau A., Schmidt K., Schrammel A., Mayer B. Aldehyde dehydrogenase-independent bioactivation of nitroglycerin in porcine and bovine blood vessels. Biochem. Pharmacol. 2015;93:440–448. doi: 10.1016/j.bcp.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ok S.H., Kwon S.C., Yeol Han J., Yu J., Shin I.W., Lee H.K., Chung Y.K., Choi M.J., Sohn J.T. Mepivacaine-induced contraction involves increased calcium sensitization mediated via Rho kinase and protein kinase C in endothelium-denuded rat aorta. Eur. J. Pharmacol. 2014;723:185–193. doi: 10.1016/j.ejphar.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 42.Taguchi K., Matsumoto T., Kamata K., Kobayashi T. Angiotensin II type 2 receptor-dependent increase in nitric oxide synthase activity in the endothelium of db/db mice is mediated via a MEK pathway. Pharmacol. Res. 2012;66:41–50. doi: 10.1016/j.phrs.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Machado N.T., Maciel P.M., Alustau M.C., Queiroz T.M., Furtado F.F., Assis V.L., Veras R.C., Araújo I.G., Athayde-Filho P.F., Medeiros I.A. Nitric oxide as a target for the hypotensive and vasorelaxing effects induced by (Z)-ethyl 12-nitrooxy-octadec-9-enoate in rats. Eur. J. Pharm. Sci. 2014;62:317–325. doi: 10.1016/j.ejps.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Celotto A.C., Capellini V.K., Restini C.B., Baldo C.F., Bendhack L.M., Evora P.R. Extracellular alkalinization induces endothelium-derived nitric oxide dependent relaxation in rat thoracic aorta. Nitric Oxide. 2010;23:269–274. doi: 10.1016/j.niox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Bonaventura D., de Lima R.G., da Silva R.S., Bendhack L.M. NO donors-relaxation is impaired in aorta from hypertensive rats due to a reduced involvement of K+ channels and sarcoplasmic reticulum Ca2+-ATPase. Life Sci. 2011;89:595–602. doi: 10.1016/j.lfs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Senejoux F., Girard C., Aisa H.A., Bakri M., Kerram P., Berthelot A., Bevalot F., Demougeot C. Vasorelaxant and hypotensive effects of a hydroalcoholic extract from the fruits of Nitraria sibirica Pall. (Nitrariaceae) J. Ethnopharmacol. 2012;141:629–634. doi: 10.1016/j.jep.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Leo C., Joshi A., Hart J., Woodman O. Endothelium-dependent nitroxyl-mediated relaxation is resistant to superoxide anion scavenging and preserved in diabetic rat aorta. Pharmacol. Res. 2012;66:383–391. doi: 10.1016/j.phrs.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Perusquia M., Espinoza J., de la Pena A. Mifepristone (RU 486) induces vasodilation and inhibits platelet aggregation: nongenomic and genomic action to cause hemorrhage. Contraception. 2011;84:169–177. doi: 10.1016/j.contraception.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Leal C.M., Pereira S.L., Kummerle A.E., Leal D.M., Tesch R., de Sant'Anna C.M., Fraga C.A., Barreiro E.J., Sudo R.T., Zapata-Sudo G. Antihypertensive profile of 2-thienyl-3,4-methylenedioxybenzoylhydrazone is mediated by activation of the A2A adenosine receptor. Eur. J. Med. Chem. 2012;55:49–57. doi: 10.1016/j.ejmech.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Zhang W., Edvinsson L., Xu C.B. Apolipoprotein B of low-density lipoprotein impairs nitric oxide-mediated endothelium-dependent relaxation in rat mesenteric arteries. Eur. J. Pharmacol. 2014;725:10–17. doi: 10.1016/j.ejphar.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Nakashima M., Shigekuni Y., Obi T., Shiraishi M., Miyamoto A., Yamasaki H., Etoh T., Iwai S. Nitric oxide-dependent hypotensive effects of wax gourd juice. J. Ethnopharmacol. 2011;138:404–407. doi: 10.1016/j.jep.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 52.Rios M.Y., López-Martínez S., López-Vallejo F., Medina-Franco J.L., Villalobos-Molina R., Ibarra-Barajas M., Navarrete-Vazquez G., Hidalgo-Figueroa S., Hernández-Abreu O., Estrada-Soto S. Vasorelaxant activity of some structurally related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by NO production: Ex vivo and in silico studies. Fitoterapia. 2012;83:1023–1029. doi: 10.1016/j.fitote.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Jerez S., Sierra L., Scacchi F., Peral de Bruno M. Hypercholesterolemia modifies angiotensin II desensitisation and cross talk between α1-adrenoceptor and angiotensin AT(1) receptor in rabbit aorta. Eur. J. Pharmacol. 2010;635:149–155. doi: 10.1016/j.ejphar.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 54.Pagan R.M., Prieto D., Hernandez M., Correa C., Garcia-Sacristan A., Benedito S., Martinez A.C. Regulation of NO-dependent acetylcholine relaxation by K+ channels and the Na+-K+ ATPase pump in porcine internal mammary artery. Eur. J. Pharmacol. 2010;641:61–66. doi: 10.1016/j.ejphar.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Sauvaget F., Mallem M.Y., Bucas V., Gogny M., Desfontis J.-C., Noireaud J. Positive influence of AT 1 receptor antagonism upon the impaired celiprolol-induced vasodilatation in aorta from spontaneously hypertensive rats. Eur. J. Pharmacol. 2010;644:169–175. doi: 10.1016/j.ejphar.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Shim H.S., Ok S.H., Lee S.H., Kwon S.C., Sohn J.T. Protein kinases participate in the contraction in response to levobupivacaine in the rat aorta. Eur. J. Pharmacol. 2012;677:131–137. doi: 10.1016/j.ejphar.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 57.Novakovic A., Marinko M., Vranic A., Jankovic G., Milojevic P., Stojanovic I., Nenezic D., Ugresic N., Kanjuh V., Yang Q., et al. Mechanisms underlying the vasorelaxation of human internal mammary artery induced by (−)-epicatechin. Eur. J. Pharmacol. 2015;762:306–312. doi: 10.1016/j.ejphar.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 58.Li X., Chen G.P., Li L., Wang K.J., Zhang B.Q., Hu S.J. Dual effects of sodium aescinate on vascular tension in rat thoracic aorta. Microvasc. Res. 2010;79:63–69. doi: 10.1016/j.mvr.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Han L., Yu Y., Sun X., Wang B. Exendin-4 directly improves endothelial dysfunction in isolated aortas from obese rats through the cAMP or AMPK–eNOS pathways. Diabetes Res. Clin. Pract. 2012;97:453–460. doi: 10.1016/j.diabres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Rodrigues S.M., Ximenes C.F., de Batista P.R., Simoes F.V., Coser P.H., Sena G.C., Podratz P.L., de Souza L.N., Vassallo D.V., Graceli J.B., et al. Tributyltin contributes in reducing the vascular reactivity to phenylephrine in isolated aortic rings from female rats. Toxicol. Lett. 2014;225:378–385. doi: 10.1016/j.toxlet.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Bankar G.R., Nayak P.G., Bansal P., Paul P., Pai K.S., Singla R.K., Bhat V.G. Vasorelaxant and antihypertensive effect of Cocos nucifera Linn. endocarp on isolated rat thoracic aorta and DOCA salt-induced hypertensive rats. J. Ethnopharmacol. 2011;134:50–54. doi: 10.1016/j.jep.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 62.Hao H.F., Liu L.M., Liu Y.Y., Liu J., Yan L., Pan C.S., Wang M.X., Wang C.S., Fan J.Y., Gao Y.S., et al. Inhibitory effect of rhynchophylline on contraction of cerebral arterioles to endothelin 1: role of rho kinase. J. Ethnopharmacol. 2014;155:147–153. doi: 10.1016/j.jep.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 63.Tao L., Hu H.S., Shen X.C. Endothelium-dependent vasodilatation effects of the essential oil from Fructus alpiniae zerumbet (EOFAZ) on rat thoracic aortic rings in vitro. Phytomedicine. 2013;20:387–393. doi: 10.1016/j.phymed.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Kalea A.Z., Clark K., Schuschke D.A., Kristo A.S., Klimis-Zacas D.J. Dietary enrichment with wild blueberries (Vaccinium angustifolium) affects the vascular reactivity in the aorta of young spontaneously hypertensive rats. J. Nutr. Biochem. 2010;21:14–22. doi: 10.1016/j.jnutbio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Wu J.H., Li Q., Wu M.Y., Guo D.J., Chen H.L., Chen S.L., Seto S.W., Au A.L., Poon C.C., Leung G.P., et al. Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. J. Nutr. Biochem. 2010;21:613–620. doi: 10.1016/j.jnutbio.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Lin A.H., Leung G.P., Leung S.W., Vanhoutte P.M., Man R.Y. Genistein enhances relaxation of the spontaneously hypertensive rat aorta by transactivation of epidermal growth factor receptor following binding to membrane estrogen receptors-α and activation of a G protein-coupled, endothelial nitric oxide synthase-dependent pathway. Pharmacol. Res. 2011;63:181–189. doi: 10.1016/j.phrs.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y., Chen Q., Sun Z., Han J., Wang L., Zheng L. Impaired capsaicin-induced relaxation in diabetic mesenteric arteries. J. Diabetes Complications. 2015;29:747–754. doi: 10.1016/j.jdiacomp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 68.Wang H.P., Lu J.F., Zhang G.L., Li X.Y., Peng H.Y., Lu Y., Zhao L., Ye Z.G., Bruce I.C., Xia Q., et al. Endothelium-dependent and -independent vasorelaxant actions and mechanisms induced by total flavonoids of Elsholtzia splendens in rat aortas. Environ. Toxicol. Pharmacol. 2014;38:453–459. doi: 10.1016/j.etap.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 69.Li Y.J., Bao J.X., Xu J.W., Murad F., Bian K. Vascular dilation by paeonol—A mechanism study. Vascul. Pharmacol. 2010;53:169–176. doi: 10.1016/j.vph.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Perez T., Lopez R.M., Lopez P., Castillo C., Castillo E.F. Lack of heterologous receptor desensitization induced by angiotensin II type 1 receptor activation in isolated normal rat thoracic aorta. Vascul. Pharmacol. 2011;54:29–35. doi: 10.1016/j.vph.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y.J., Wang L., Zhou G.Y., Yu X.L., Zhang Y.H., Hu N., Li Q.Q., Chen C., Qing C., Liu Y.T., et al. Scutellarin attenuates endothelium-dependent aasodilation impairment induced by hypoxia reoxygenation, through regulating the PKG signaling pathway in rat coronary artery. Chin. J. Nat. Med. 2015;13:264–273. doi: 10.1016/S1875-5364(15)30013-3. [DOI] [PubMed] [Google Scholar]
- 72.Araujo A.V., Ferezin C.Z., Rodrigues G.J., Lunardi C.N., Vercesi J.A., Grando M.D., Bonaventura D., Bendhack L.M. Prostacyclin, not only nitric oxide, is a mediator of the vasorelaxation induced by acetylcholine in aortas from rats submitted to cecal ligation and perforation (CLP) Vascul. Pharmacol. 2011;54:44–51. doi: 10.1016/j.vph.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Shen M., Zhao L., Wu R.X., Yue S.Q., Pei J.M. The vasorelaxing effect of resveratrol on abdominal aorta from rats and its underlying mechanisms. Vascul. Pharmacol. 2013;58:64–70. doi: 10.1016/j.vph.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Lee K.Y., Choi H.C. Acetylcholine-induced AMP-activated protein kinase activation attenuates vasoconstriction through an LKB1-dependent mechanism in rat aorta. Vascul. Pharmacol. 2013;59:96–102. doi: 10.1016/j.vph.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 75.Perez-Aso M., Flacco N., Carpena N., Montesinos M.C., D'Ocon P., Ivorra M.D. β-Adrenoceptors differentially regulate vascular tone and angiogenesis of rat aorta via ERK1/2 and p38. Vascul. Pharmacol. 2014;61:80–89. doi: 10.1016/j.vph.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharya I., Damjanovic M., Dominguez A.P., Haas E. Inhibition of activated ERK1/2 and JNKs improves vascular function in mouse aortae in the absence of nitric oxide. Eur. J. Pharmacol. 2011;658:22–27. doi: 10.1016/j.ejphar.2010.09.053. [DOI] [PubMed] [Google Scholar]
- 77.El-Gowelli H.M., El-Gowilly S.M., Elsalakawy L.K., El-Mas M.M. Nitric oxide synthase/K+ channel cascade triggers the adenosine A2B receptor-sensitive renal vasodilation in female rats. Eur. J. Pharmacol. 2013;702:116–125. doi: 10.1016/j.ejphar.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 78.Sun X., Hou N., Han F., Guo Y., Hui Z., Du G., Zhang Y. Effect of high free fatty acids on the anti-contractile response of perivascular adipose tissue in rat aorta. J. Mol. Cell. Cardiol. 2013;63:169–174. doi: 10.1016/j.yjmcc.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Gallo L.C., Davel A.P., Xavier F.E., Rossoni L.V. Time-dependent increases in ouabain-sensitive Na+, K+-ATPase activity in aortas from diabetic rats: The role of prostanoids and protein kinase C. Life Sci. 2010;87:302–308. doi: 10.1016/j.lfs.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Horta C., Rezende B., Oliveira-Mendes B., Carmo A., Capettini L., Silva J., Gomes M., Chávez-Olórtegui C., Bravo C., Lemos V. ADP is a vasodilator component from Lasiodora sp. mygalomorph spider venom. Toxicon. 2013;72:102–112. doi: 10.1016/j.toxicon.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Taguchi K., Kobayashi T., Takenouchi Y., Matsumoto T., Kamata K. Angiotensin II causes endothelial dysfunction via the GRK2/Akt/eNOS pathway in aortas from a murine type 2 diabetic model. Pharmacol. Res. 2011;64:535–546. doi: 10.1016/j.phrs.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Jin S.N., Wen J.F., Li X., Kang D.G., Lee H.S., Cho K.W. The mechanism of vasorelaxation induced by ethanol extract of Sophora flavescens in rat aorta. J. Ethnopharmacol. 2011;137:547–552. doi: 10.1016/j.jep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Gaete P.S., Lillo M.A., Ardiles N.M., Perez F.R., Figueroa X.F. Ca2+-activated K+ channels of small and intermediate conductance control eNOS activation through NAD(P)H oxidase. Free Radic. Biol. Med. 2012;52:860–870. doi: 10.1016/j.freeradbiomed.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 84.Silveira E.A., Siman F.D., de Oliveira Faria T., Vescovi M.V., Furieri L.B., Lizardo J.H., Stefanon I., Padilha A.S., Vassallo D.V. Low-dose chronic lead exposure increases systolic arterial pressure and vascular reactivity of rat aortas. Free Radic. Biol. Med. 2014;67:366–376. doi: 10.1016/j.freeradbiomed.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 85.Dongmo A.B., Nkeng-Efouet P.A., Devkota K.P., Wegener J.W., Sewald N., Wagner H., Vierling W. Tetra-acetylajugasterone a new constituent of Vitex cienkowskii with vasorelaxant activity. Phytomedicine. 2014;21:787–792. doi: 10.1016/j.phymed.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Z., Sun C., Tilley S.L., Mustafa S.J. Mechanisms underlying uridine adenosine tetraphosphate-induced vascular contraction in mouse aorta: Role of thromboxane and purinergic receptors. Vascul. Pharmacol. 2015;73:78–85. doi: 10.1016/j.vph.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ling W.C., Lau Y.S., Murugan D.D., Vanhoutte P.M., Mustafa M.R. Sodium nitrite causes relaxation of the isolated rat aorta: By stimulating both endothelial NO synthase and activating soluble guanylyl cyclase in vascular smooth muscle. Vascul. Pharmacol. 2015;74:87–92. doi: 10.1016/j.vph.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 88.Al-Nakkash L., Martin J.B., Petty D., Lynch S.M., Hamrick C., Lucy D., Robinson J., Peterson A., Rubin L.J., Broderick T.L. Dietary genistein induces sex-dependent effects on murine body weight, serum profiles, and vascular function of thoracic aortae. Gend. Med. 2012;9:295–308. doi: 10.1016/j.genm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Sélley E., Molnár G.A., Kun S., Szijártó I.A., Laczy B., Kovács T., Fülöp F., Wittmann I. Complex vasoactivity of liraglutide. Contribution of three gasotransmitters. Artery Res. 2015;11:1–9. doi: 10.1016/j.artres.2015.04.001. [DOI] [Google Scholar]
- 90.Choi S., Jung W.S., Cho N.S., Ryu K.H., Jun J.Y., Shin B.C., Chung J.H., Yeum C.H. Mechanisms of phytoestrogen biochanin A-induced vasorelaxation in renovascular hypertensive rats. Kidney Res. Clin. Pract. 2014;33:181–186. doi: 10.1016/j.krcp.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ntchapda F., Talla E., Sakava P., Tanzi F., Fohouo F.-N.T., Tanyi J.M., Dimo T. Nitric oxide-dependent vasodilation and Ca 2+ signalling induced by erythrodiol in rat aorta. Asian Pac. J. Trop. Dis. 2015;5:S214–S223. doi: 10.1016/S2222-1808(15)60892-1. [DOI] [Google Scholar]
- 92.Nsuadi Manga F., El Khattabi C., Fontaine J., Berkenboom G., Duez P., Noyon C., van Antwerpen P., Lami Nzunzu J., Pochet S. Vasorelaxant and antihypertensive effects of methanolic extracts from Hymenocardia acida Tul. J. Ethnopharmacol. 2013;146:623–631. doi: 10.1016/j.jep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Paredes-Carbajal M.C., Monsalvo I., Hernandez-Diaz C., Regla I., Demare P., Mascher D. Effects of ranolazine on vasomotor responses of rat aortic rings. Arch. Med. Res. 2013;44:8–12. doi: 10.1016/j.arcmed.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Li X., Kim H.Y., Cui H.Z., Cho K.W., Kang D.G., Lee H.S. Water extract of Zanthoxylum piperitum induces vascular relaxation via endothelium-dependent NO-cGMP signaling. J. Ethnopharmacol. 2010;129:197–202. doi: 10.1016/j.jep.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 95.Jin S.N., Wen J.F., Kim H.Y., Kang D.G., Lee H.S., Cho K.W. Vascular relaxation by ethanol extract of Xanthoceras sorbifolia via Akt- and SOCE-eNOS-cGMP pathways. J. Ethnopharmacol. 2010;132:240–245. doi: 10.1016/j.jep.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 96.Kim H.Y., Oh H., Li X., Cho K.W., Kang D.G., Lee H.S. Ethanol extract of seeds of Oenothera odorata induces vasorelaxation via endothelium-dependent NO-cGMP signaling through activation of Akt-eNOS-sGC pathway. J. Ethnopharmacol. 2011;133:315–323. doi: 10.1016/j.jep.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 97.Nsuadi Manga F., El Khattabi C., Fontaine J., Berkenboom G., Duez P., Lami Nzunzu J., Pochet S. Vascular effects and antioxidant activity of two Combretum species from Democratic Republic of Congo. J. Ethnopharmacol. 2012;142:194–200. doi: 10.1016/j.jep.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 98.Bernardes M.J., de Carvalho F.S., Lima Silveira L., de Paula J.R., Bara M.T., Garrote C.F., Pedrino G.R., Rocha M.L. Hypotensive effect of Aspidosperma subincanum Mart. in rats and its mechanism of vasorelaxation in isolated arteries. J. Ethnopharmacol. 2013;145:227–232. doi: 10.1016/j.jep.2012.10.057. [DOI] [PubMed] [Google Scholar]
- 99.Senejoux F., Demougeot C., Cuciureanu M., Miron A., Cuciureanu R., Berthelot A., Girard-Thernier C. Vasorelaxant effects and mechanisms of action of Heracleum sphondylium L. (Apiaceae) in rat thoracic aorta. J. Ethnopharmacol. 2013;147:536–539. doi: 10.1016/j.jep.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 100.Cuinas A., Elies J., Orallo F., Campos-Toimil M. Cyclic AMP relaxation of rat aortic smooth muscle is mediated in part by decrease of depletion of intracellular Ca2+ stores and inhibition of capacitative calcium entry. Vascul. Pharmacol. 2013;58:98–104. doi: 10.1016/j.vph.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Morales V., Cuinas A., Elies J., Campos-Toimil M. PKA and Epac activation mediates cAMP-induced vasorelaxation by increasing endothelial NO production. Vascul. Pharmacol. 2014;60:95–101. doi: 10.1016/j.vph.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 102.Deng Y., Ng E.S., Kwan Y.W., Lau C.B., Cheung D.W., Koon J.C., Zhang Z., Zuo Z., Leung P.C., Fung K.P., et al. Cerebral vasodilator properties of Danshen and Gegen: A study of their combined efficacy and mechanisms of actions. Phytomedicine. 2014;21:391–399. doi: 10.1016/j.phymed.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 103.Ng H.K., Poh T.F., Lam S.K., Hoe S.Z. Potassium channel openers and prostacyclin play a crucial role in mediating the vasorelaxant activity of Gynura procumbens. BMC Complement. Altern. Med. 2013;13:188. doi: 10.1186/1472-6882-13-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang H.-Q., Liu Y.-Y., Li Y.-W., Li L., Cui Z.-Q. Effects of total alkaloids in Buxus microphylla leaves on aorta smooth muscle of rats and their mechanisms. Chin. Herbal Med. 2012;4:136–141. [Google Scholar]
- 105.Potje S.R., Munhoz F.C., Perassa L.A., Graton M.E., Pereira A.A., Nakamune A.C., da Silva R.S., Bendhack L.M., Sumida D.H., Antoniali C. Mechanisms underlying the hypotensive and vasodilator effects of Ru(terpy)(bdq)NO]3+, a nitric oxide donor, differ between normotensive and spontaneously hypertensive rats. Eur. J. Pharmacol. 2014;741:222–229. doi: 10.1016/j.ejphar.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V., Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: Role of epigenetic histone modifications. Biochem. Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 107.Yang Z., Zhang Y., Meng Z. The vasodilator mechanisms of sodium metabisulfite on precontracted isolated aortic rings in rats: signal transduction pathways and ion channels. Food Chem. Toxicol. 2012;50:3114–3119. doi: 10.1016/j.fct.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 108.Senejoux F., Girard C., Kerram P., Aisa H.A., Berthelot A., Bevalot F., Demougeot C. Mechanisms of vasorelaxation induced by Ziziphora clinopodioides Lam. (Lamiaceae) extract in rat thoracic aorta. J. Ethnopharmacol. 2010;132:268–273. doi: 10.1016/j.jep.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 109.Monteiro F.S., Silva A.C., Martins I.R., Correia A.C., Basilio I.J., Agra M.F., Bhattacharyya J., Silva B.A. Vasorelaxant action of the total alkaloid fraction obtained from Solanum paludosum Moric. (Solanaceae) involves NO/cGMP/PKG pathway and potassium channels. J. Ethnopharmacol. 2012;141:895–900. doi: 10.1016/j.jep.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 110.Park J.Y., Choi Y.W., Yun J.W., Bae J.U., Seo K.W., Lee S.J., Park S.Y., Kim C.D. Gomisin J from Schisandra chinensis induces vascular relaxation via activation of endothelial nitric oxide synthase. Vascul. Pharmacol. 2012;57:124–130. doi: 10.1016/j.vph.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 111.Garcia-Prieto C.F., Pulido-Olmo H., Ruiz-Hurtado G., Gil-Ortega M., Aranguez I., Rubio M.A., Ruiz-Gayo M., Somoza B., Fernandez-Alfonso M.S. Mild caloric restriction reduces blood pressure and activates endothelial AMPK-PI3K-Akt-eNOS pathway in obese Zucker rats. Vascul. Pharmacol. 2015;65–66:3–12. doi: 10.1016/j.vph.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 112.Choudhury S., Kannan K., Pule Addison M., Darzi S.A., Singh V., Singh T.U., Thangamalai R., Dash J.R., Parida S., Debroy B., et al. Combined treatment with atorvastatin and imipenem improves survival and vascular functions in mouse model of sepsis. Vascul. Pharmacol. 2015;71:139–150. doi: 10.1016/j.vph.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 113.Masszi G., Novak A., Tarszabo R., Horvath E.M., Buday A., Ruisanchez E., Tokes A.M., Sara L., Benko R., Nadasy G.L., et al. Effects of vitamin D3 derivative—Calcitriol on pharmacological reactivity of aortic rings in a rodent PCOS model. Pharmacol. Rep. 2013;65:476–483. doi: 10.1016/S1734-1140(13)71023-5. [DOI] [PubMed] [Google Scholar]
- 114.Kazmierczak P.A., Dobaczewski M.P., Przygodzki T., Carsky J., Watala C. β-Resorcylidene aminoguanidine (RAG) dilates coronary arteries in an endothelium-independent manner. Pharmacol. Rep. 2015;67:631–635. doi: 10.1016/j.pharep.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 115.Munin J., Quezada E., Cuinas A., Campos-Toimil M., Uriarte E., Santana L., Vina D. Synthesis, biological evaluation and structure-activity relationships of new phthalazinedione derivatives with vasorelaxant activity. Eur. J. Med. Chem. 2014;82:407–417. doi: 10.1016/j.ejmech.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 116.Meyer M.R., Barton M., Prossnitz E.R. Functional heterogeneity of NADPH oxidase-mediated contractions to endothelin with vascular aging. Life Sci. 2014;118:226–231. doi: 10.1016/j.lfs.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maia-Joca R.P., Joca H.C., Ribeiro F.J., do Nascimento R.V., Silva-Alves K.S., Cruz J.S., Coelho-de-Souza A.N., Leal-Cardoso J.H. Investigation of terpinen-4-ol effects on vascular smooth muscle relaxation. Life Sci. 2014;115:52–58. doi: 10.1016/j.lfs.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 118.Kamiya T., Nagaoka T., Omae T., Yoshioka T., Ono S., Tanano I., Yoshida A. Role of Ca2+-dependent and Ca2+-sensitive mechanisms in sphingosine 1-phosphate-induced constriction of isolated porcine retinal arterioles in vitro. Exp. Eye Res. 2014;121:94–101. doi: 10.1016/j.exer.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 119.Fajemiroye J.O., Amaral N.O., da Silva E.F., Galdino P.M., de Oliveira T.S., Ghedini P.C., Zjawiony J.K., Costa E.A., Pedrino G.R., Menegatti R. Hypotensive and antihypertensive potential of 4-[(1-phenyl-1H-pyrazol-4-yl) methyl]1-piperazine carboxylic acid ethyl ester: A piperazine derivative. Life Sci. 2014;112:90–96. doi: 10.1016/j.lfs.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 120.Tom E.N., Girard-Thernier C., Martin H., Dimo T., Alvergnas M., Nappey M., Berthelot A., Demougeot C. Treatment with an extract of Terminalia superba Engler & Diels decreases blood pressure and improves endothelial function in spontaneously hypertensive rats. J. Ethnopharmacol. 2014;151:372–379. doi: 10.1016/j.jep.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 121.Boonla O., Kukongviriyapan U., Pakdeechote P., Kukongviriyapan V., Pannangpetch P., Prachaney P., Greenwald S.E. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44–53. doi: 10.1016/j.niox.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 122.Inoue Y., Nakahara K., Maruyama K., Suzuki Y., Hayashi Y., Kangawa K., Murakami N. Central and peripheral des-acyl ghrelin regulates body temperature in rats. Biochem. Biophys. Res. Commun. 2013;430:278–283. doi: 10.1016/j.bbrc.2012.10.137. [DOI] [PubMed] [Google Scholar]
- 123.Pereira S.L., Kummerle A.E., Fraga C.A., Barreiro E.J., Rocha Nde N., Ferraz E.B., do Nascimento J.H., Sudo R.T., Zapata-Sudo G. A novel Ca2+ channel antagonist reverses cardiac hypertrophy and pulmonary arteriolar remodeling in experimental pulmonary hypertension. Eur. J. Pharmacol. 2013;702:316–322. doi: 10.1016/j.ejphar.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 124.Priestley J.R., Buelow M.W., McEwen S.T., Weinberg B.D., Delaney M., Balus S.F., Hoeppner C., Dondlinger L., Lombard J.H. Reduced angiotensin II levels cause generalized vascular dysfunction via oxidant stress in hamster cheek pouch arterioles. Microvasc. Res. 2013;89:134–145. doi: 10.1016/j.mvr.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Babacanoglu C., Yildirim N., Sadi G., Pektas M.B., Akar F. Resveratrol prevents high-fructose corn syrup-induced vascular insulin resistance and dysfunction in rats. Food Chem. Toxicol. 2013;60:160–167. doi: 10.1016/j.fct.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 126.Sinagra T., Tamburella A., Urso V., Siarkos I., Drago F., Bucolo C., Salomone S. Reversible inhibition of vasoconstriction by thiazolidinediones related to PI3K/Akt inhibition in vascular smooth muscle cells. Biochem. Pharmacol. 2013;85:551–559. doi: 10.1016/j.bcp.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 127.Brito T.S., Lima F.J., Aragao K.S., de Siqueira R.J., Sousa P.J., Maia J.G., Filho J.D., Lahlou S., Magalhaes P.J. The vasorelaxant effects of 1-nitro-2-phenylethane involve stimulation of the soluble guanylate cyclase-cGMP pathway. Biochem. Pharmacol. 2013;85:780–788. doi: 10.1016/j.bcp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 128.Simões M.R., Furieri L.B., Forechi L., Baldo M.P., Rodrigues S.L., Salaices M., Vassallo D.V., Mill J.G. High salt intake does not produce additional impairment in the coronary artery relaxation of spontaneously hypertensive aged rats. Food Chem. Toxicol. 2013;58:193–197. doi: 10.1016/j.fct.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 129.Roberts R.E., Allen S., Chang A.P., Henderson H., Hobson G.C., Karania B., Morgan K.N., Pek A.S., Raghvani K., Shee C.Y., et al. Distinct mechanisms of relaxation to bioactive components from chamomile species in porcine isolated blood vessels. Toxicol. Appl. Pharmacol. 2013;272:797–805. doi: 10.1016/j.taap.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 130.Centeno J.M., Marrachelli V.G., Miranda L., Castello-Ruiz M., Burguete M.C., Jover-Mengual T., Salom J.B., Torregrosa G., Miranda F.J., Alborch E. Involvement of prostacyclin and potassium channels in the diabetes-induced hyporeactivity of the rabbit carotid artery to B-type natriuretic peptide. Eur. J. Pharmacol. 2013;701:159–167. doi: 10.1016/j.ejphar.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 131.Oh K.S., Oh B.K., Park C.H., Seo H.W., Kang N.S., Lee J.H., Lee J.S., Ho Lee B. Cardiovascular effects of a novel selective Rho kinase inhibitor, 2-(1H-indazole-5-yl)amino-4-methoxy-6-piperazino triazine (DW1865) Eur. J. Pharmacol. 2013;702:218–226. doi: 10.1016/j.ejphar.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 132.Kriska T., Cepura C., Siangjong L., Wan T.C., Auchampach J.A., Shaish A., Haratz D., Kumar G., Falck J.R., Gauthier K.M., et al. Effect of human 15-lipoxygenase-1 metabolites on vascular function in mouse mesenteric arteries and hearts. Prostaglandins Other Lipid Mediat. 2013;106:8–15. doi: 10.1016/j.prostaglandins.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nofal Z.M., Srour A.M., El-Eraky W.I., Saleh D.O., Girgis A.S. Rational design, synthesis and QSAR study of vasorelaxant active 3-pyridinecarbonitriles incorporating 1H-benzimidazol-2-yl function. Eur. J. Med. Chem. 2013;63:14–21. doi: 10.1016/j.ejmech.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 134.Wang T.T., Zhou G.H., Kho J.H., Sun Y.Y., Wen J.F., Kang D.G., Lee H.S., Cho K.W., Jin S.N. Vasorelaxant action of an ethylacetate fraction of Euphorbia humifusa involves NO-cGMP pathway and potassium channels. J. Ethnopharmacol. 2013;148:655–663. doi: 10.1016/j.jep.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 135.Gortan Cappellari G., Losurdo P., Mazzucco S., Panizon E., Jevnicar M., Macaluso L., Fabris B., Barazzoni R., Biolo G., Carretta R., et al. Treatment with n-3 polyunsaturated fatty acids reverses endothelial dysfunction and oxidative stress in experimental menopause. J. Nutr. Biochem. 2013;24:371–379. doi: 10.1016/j.jnutbio.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 136.Martelli A., Testai L., Breschi M.C., Lawson K., McKay N.G., Miceli F., Taglialatela M., Calderone V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol. Res. 2013;70:27–34. doi: 10.1016/j.phrs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 137.Sukumaran S.V., Singh T.U., Parida S., Narasimha Reddy Ch E., Thangamalai R., Kandasamy K., Singh V., Mishra S.K. TRPV4 channel activation leads to endothelium-dependent relaxation mediated by nitric oxide and endothelium-derived hyperpolarizing factor in rat pulmonary artery. Pharmacol. Res. 2013;78:18–27. doi: 10.1016/j.phrs.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 138.Yan J., Chen R., Liu P., Gu Y. Docosahexaenoic acid attenuates hypoxic pulmonary vasoconstriction by activating the large conductance Ca2+-activated K+ currents in pulmonary artery smooth muscle cells. Pulm. Pharmacol. Ther. 2014;28:9–16. doi: 10.1016/j.pupt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 139.Cekic E.G., Soydan G., Guler S., Babaoglu M.O., Tuncer M. Propranolol-induced relaxation in the rat basilar artery. Vascul. Pharmacol. 2013;58:307–312. doi: 10.1016/j.vph.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 140.Dalaklioglu S., Tasatargil A., Kale S., Tanriover G., Dilmac S., Erin N. Metastatic breast carcinoma induces vascular endothelial dysfunction in Balb-c mice: Role of the tumor necrosis factor-α and NADPH oxidase. Vascul. Pharmacol. 2013;59:103–111. doi: 10.1016/j.vph.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 141.El-Kashef D.H., El-Agamy D.S., Gamil N.M. Protective effects of hydrogen sulfide against high glucose induced-endothelial dysfunction: An in vitro study. J. Taibah Univ. Sci. 2013;7:97–104. doi: 10.1016/j.jtusci.2013.05.002. [DOI] [Google Scholar]
- 142.Senbel A.M., Omar A.G., Abdel-Moneim L.M., Mohamed H.F., Daabees T.T. Evaluation of l-arginine on kidney function and vascular reactivity following ischemic injury in rats: protective effects and potential interactions. Pharmacol. Rep. 2014;66:976–983. doi: 10.1016/j.pharep.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 143.Li L., Yang H.G., Yuan T.Y., Zhao Y., Du G.H. Rho kinase inhibition activity of pinocembrin in rat aortic rings contracted by angiotensin II. Chin. J. Nat. Med. 2013;11:258–263. doi: 10.3724/SP.J.1009.2013.00258. [DOI] [PubMed] [Google Scholar]
- 144.McCormick C., Jones R.L., Kennedy S., Wadsworth R.M. Activation of prostanoid EP receptors by prostacyclin analogues in rabbit iliac artery: implications for anti-restenotic potential. Eur. J. Pharmacol. 2010;641:160–167. doi: 10.1016/j.ejphar.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 145.Takebayashi K., Sohma R., Aso Y., Inukai T. Effects of retinol binding protein-4 on vascular endothelial cells. Biochem. Biophys. Res. Commun. 2011;408:58–64. doi: 10.1016/j.bbrc.2011.03.116. [DOI] [PubMed] [Google Scholar]
- 146.Matsuo Y., Kuwabara M., Tanaka-Totoribe N., Kanai T., Nakamura E., Gamoh S., Suzuki A., Asada Y., Hisa H., Yamamoto R. The defective protein level of myosin light chain phosphatase (MLCP) in the isolated saphenous vein, as a vascular conduit in coronary artery bypass grafting (CABG), harvested from patients with diabetes mellitus (DM) Biochem. Biophys. Res. Commun. 2011;412:323–327. doi: 10.1016/j.bbrc.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 147.Giustarini D., Tsikas D., Rossi R. Study of the effect of thiols on the vasodilatory potency of S-nitrosothiols by using a modified aortic ring assay. Toxicol. Appl. Pharmacol. 2011;256:95–102. doi: 10.1016/j.taap.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 148.Lu C., Su L.Y., Lee R.M., Gao Y.J. Alterations in perivascular adipose tissue structure and function in hypertension. Eur. J. Pharmacol. 2011;656:68–73. doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 149.Sapa J., Kubacka M. The possible mechanism of hypotensive activity of some pyrrolidin-2-one derivatives with antagonist properties at α1-adrenoceptors. Eur. J. Pharmacol. 2011;673:40–48. doi: 10.1016/j.ejphar.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 150.Lamarre N.S., Parry T., Tallarida R.J. On the quantitation of an agonist with dual but opposing components of action: Application to vascular endothelial relaxation. Eur. J. Pharmacol. 2011;670:204–207. doi: 10.1016/j.ejphar.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Van Drongelen J., Pertijs J., Wouterse A., Hermsen R., Sweep F.C., Lotgering F.K., Smits P., Spaanderman M.E. Contribution of different local vascular responses to mid-gestational vasodilation. Am. J. Obstet. Gynecol. 2011;205:155.e12–155.e17. doi: 10.1016/j.ajog.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 152.Yamazaki T., Anraku T., Matsuzawa S. Ibudilast, a mixed PDE3/4 inhibitor, causes a selective and nitric oxide/cGMP-independent relaxation of the intracranial vertebrobasilar artery. Eur. J. Pharmacol. 2011;650:605–611. doi: 10.1016/j.ejphar.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 153.Fiorim J., Ribeiro R.F., Jr., Azevedo B.F., Simoes M.R., Padilha A.S., Stefanon I., Alonso M.J., Salaices M., Vassallo D.V. Activation of K+ channels and Na+/K+ ATPase prevents aortic endothelial dysfunction in 7-day lead-treated rats. Toxicol. Appl. Pharmacol. 2012;262:22–31. doi: 10.1016/j.taap.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 154.Agbor L.N., Walsh M.T., Boberg J.R., Walker M.K. Elevated blood pressure in cytochrome P4501A1 knockout mice is associated with reduced vasodilation to omega-3 polyunsaturated fatty acids. Toxicol. Appl. Pharmacol. 2012;264:351–360. doi: 10.1016/j.taap.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zapata-Sudo G., Pontes L.B., da Silva J.S., Lima L.M., Nunes I.K., Barreiro E.J., Sudo R.T. Benzenesulfonamide attenuates monocrotaline-induced pulmonary arterial hypertension in a rat model. Eur. J. Pharmacol. 2012;690:176–182. doi: 10.1016/j.ejphar.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 156.Sara L., Antal P., Masszi G., Buday A., Horvath E.M., Hamar P., Monos E., Nadasy G.L., Varbiro S. Arteriolar insulin resistance in a rat model of polycystic ovary syndrome. Fertil. Steril. 2012;97:462–468. doi: 10.1016/j.fertnstert.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 157.Alvarez-Medina D.I., Hernandez A., Orozco C. Endothelial hyperpolarizing factor increases acetylcholine-induced vasodilatation in pulmonary hypertensive broilers arterial rings. Res. Vet. Sci. 2012;92:1–6. doi: 10.1016/j.rvsc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 158.Liao S.B., O W.S., Tang F. Adrenomedullin inhibits norepinephrine-induced contraction of rat seminal vesicle. Urology. 2012;80:224.e1–224.e5. doi: 10.1016/j.urology.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 159.Meng Z., Yang Z., Li J., Zhang Q. The vasorelaxant effect and its mechanisms of sodium bisulfite as a sulfur dioxide donor. Chemosphere. 2012;89:579–584. doi: 10.1016/j.chemosphere.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 160.Xie W., Zhang X., Wang T., Hu J. Botany, traditional uses, phytochemistry and pharmacology of Apocynum venetum L. (Luobuma): A review. J. Ethnopharmacol. 2012;141:1–8. doi: 10.1016/j.jep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 161.Quintela A.M., Jimenez R., Gomez-Guzman M., Zarzuelo M.J., Galindo P., Sanchez M., Vargas F., Cogolludo A., Tamargo J., Perez-Vizcaino F., et al. Activation of peroxisome proliferator-activated receptor-β/-δ (PPARβ/δ) prevents endothelial dysfunction in type 1 diabetic rats. Free Radic. Biol. Med. 2012;53:730–741. doi: 10.1016/j.freeradbiomed.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 162.Del Bo C., Kristo A.S., Kalea A.Z., Ciappellano S., Riso P., Porrini M., Klimis-Zacas D. The temporal effect of a wild blueberry (Vaccinium angustifolium)-enriched diet on vasomotor tone in the Sprague-Dawley rat. Nutr. Metab. Cardiovasc. Dis. 2012;22:127–132. doi: 10.1016/j.numecd.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 163.Jerez S., Sierra L., de Bruno M.P. 17-Octadecynoic acid improves contractile response to angiotensin II by releasing vasocontrictor prostaglandins. Prostaglandins Other Lipid Mediat. 2012;97:36–42. doi: 10.1016/j.prostaglandins.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 164.Matsumoto T., Szasz T., Tostes R.C., Webb R.C. Impaired β-adrenoceptor-induced relaxation in small mesenteric arteries from DOCA-salt hypertensive rats is due to reduced K(Ca) channel activity. Pharmacol. Res. 2012;65:537–545. doi: 10.1016/j.phrs.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pan C., Huo Y., An X., Singh G., Chen M., Yang Z., Pu J., Li J. Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vascul. Pharmacol. 2012;56:150–158. doi: 10.1016/j.vph.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 166.Aloysius U.I., Achike F.I., Mustafa M.R. Mechanisms underlining gender differences in Phenylephrine contraction of normoglycaemic and short-term Streptozotocin-induced diabetic WKY rat aorta. Vascul. Pharmacol. 2012;57:81–90. doi: 10.1016/j.vph.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 167.Pelham C.J., Ketsawatsomkron P., Groh S., Grobe J.L., de Lange W.J., Ibeawuchi S.R., Keen H.L., Weatherford E.T., Faraci F.M., Sigmund C.D. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARgamma and RhoA/Rho-kinase. Cell Metab. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beleznai T., Bagi Z. Activation of hexosamine pathway impairs nitric oxide (NO)-dependent arteriolar dilations by increased protein O-GlcNAcylation. Vascul. Pharmacol. 2012;56:115–121. doi: 10.1016/j.vph.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen B., Shi L., Yu X., Sun J., Zhang H., Wang S., Fang L., Du G. Differential effects of Rho-kinase inhibitor and angiotensin II type-1 receptor antagonist on the vascular function in hypertensive rats induced by chronic l-NAME treatment. Acta Pharm. Sin. B. 2012;2:450–458. doi: 10.1016/j.apsb.2012.04.002. [DOI] [Google Scholar]
- 170.Haines R.J., Corbin K.D., Pendleton L.C., Meininger C.J., Eichler D.C. Insulin transcriptionally regulates argininosuccinate synthase to maintain vascular endothelial function. Biochem. Biophys. Res. Commun. 2012;421:9–14. doi: 10.1016/j.bbrc.2012.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Davel A.P., Lemos M., Pastro L.M., Pedro S.C., de Andre P.A., Hebeda C., Farsky S.H., Saldiva P.H., Rossoni L.V. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology. 2012;295:39–46. doi: 10.1016/j.tox.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 172.Toba H., Tojo C., Wang J., Noda K., Kobara M., Nakata T. Telmisartan inhibits vascular dysfunction and inflammation via activation of peroxisome proliferator-activated receptor-gamma in subtotal nephrectomized rat. Eur. J. Pharmacol. 2012;685:91–98. doi: 10.1016/j.ejphar.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 173.Murakami K., Inoue N., Fuchikami C., Tajima K., Hashino A., Fukui H., Noda K., Oka M. Blockade of voltage-gated calcium channel Cav1.2 and α1-adrenoceptors increases vertebral artery blood flow induced by the antivertigo agent difenidol. Eur. J. Pharmacol. 2012;689:165–171. doi: 10.1016/j.ejphar.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 174.Sathishkumar K., Elkins R., Yallampalli U., Balakrishnan M., Yallampalli C. Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Hum. Dev. 2011;87:407–414. doi: 10.1016/j.earlhumdev.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Malakul W., Thirawarapan S., Ingkaninan K., Sawasdee P. Effects of Kaempferia parviflora Wall. Ex Baker on endothelial dysfunction in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2011;133:371–377. doi: 10.1016/j.jep.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 176.Malakul W., Ingkaninan K., Sawasdee P., Woodman O.L. The ethanolic extract of Kaempferia parviflora reduces ischaemic injury in rat isolated hearts. J. Ethnopharmacol. 2011;137:184–191. doi: 10.1016/j.jep.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 177.Bertin R., Garcia-Argaez A., Martinez-Vazquez M., Froldi G. Age-dependent vasorelaxation of Casimiroa edulis and Casimiroa pubescens extracts in rat caudal artery in vitro. J. Ethnopharmacol. 2011;137:934–936. doi: 10.1016/j.jep.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 178.Witting P.K., Song C., Hsu K., Hua S., Parry S.N., Aran R., Geczy C., Freedman S.B. The acute-phase protein serum amyloid A induces endothelial dysfunction that is inhibited by high-density lipoprotein. Free Radic. Biol. Med. 2011;51:1390–1398. doi: 10.1016/j.freeradbiomed.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 179.Matsuo H., Okamoto R., Zaima K., Hirasawa Y., Ismail I.S., Lajis N.H., Morita H. New vasorelaxant indole alkaloids, villocarines A–D from Uncaria villosa. Bioorg. Med. Chem. 2011;19:4075–4079. doi: 10.1016/j.bmc.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 180.Morita H., Zaima K., Koga I., Saito A., Tamamoto H., Okazaki H., Kaneda T., Hashimoto T., Asakawa Y. Vasorelaxant effects of macrocyclic bis(bibenzyls) from liverworts. Bioorg. Med. Chem. 2011;19:4051–4056. doi: 10.1016/j.bmc.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 181.Monroy-Ruiz J., Sevilla M.A., Carron R., Montero M.J. Astaxanthin-enriched-diet reduces blood pressure and improves cardiovascular parameters in spontaneously hypertensive rats. Pharmacol. Res. 2011;63:44–50. doi: 10.1016/j.phrs.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 182.Siarkos I., Urso V., Sinagra T., Drago F., Salomone S. Endothelium-dependent vasomotor effects of telmisartan in isolated rat femoral arteries. Pharmacol. Res. 2011;63:199–206. doi: 10.1016/j.phrs.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 183.Wojcicka G., Jamroz-Wisniewska A., Atanasova P., Chaldakov G.N., Chylinska-Kula B., Beltowski J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol. Res. 2011;63:68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 184.Marrachelli V.G., Miranda F.J., Centeno J.M., Miranda I., Castello-Ruiz M., Burguete M.C., Jover-Mengual T., Salom J.B., Torregrosa G., Alborch E. Mechanisms underlying the diabetes-induced hyporeactivity of the rabbit carotid artery to atrial natriuretic peptide. Pharmacol. Res. 2011;63:190–198. doi: 10.1016/j.phrs.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 185.Sciorati C., Miglietta D., Buono R., Pisa V., Cattaneo D., Azzoni E., Brunelli S., Clementi E. A dual acting compound releasing nitric oxide (NO) and ibuprofen, NCX 320, shows significant therapeutic effects in a mouse model of muscular dystrophy. Pharmacol. Res. 2011;64:210–217. doi: 10.1016/j.phrs.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cantu-Medellin N., Vitturi D.A., Rodriguez C., Murphy S., Dorman S., Shiva S., Zhou Y., Jia Y., Palmer A.F., Patel R.P. Effects of T- and R-state stabilization on deoxyhemoglobin-nitrite reactions and stimulation of nitric oxide signaling. Nitric Oxide. 2011;25:59–69. doi: 10.1016/j.niox.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pereira A.D.C., Ford P.C., da Silva R.S., Bendhack L.M. Ruthenium-nitrite complex as pro-drug releases NO in a tissue and enzyme-dependent way. Nitric Oxide. 2011;24:192–198. doi: 10.1016/j.niox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 188.Mei Y., Jin H., Tian W., Wang H., Wang H., Zhao Y., Zhang Z., Meng F. Urantide alleviates monocrotaline induced pulmonary arterial hypertension in Wistar rats. Pulm. Pharmacol. Ther. 2011;24:386–393. doi: 10.1016/j.pupt.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 189.Li Q., Chen Y., Sun L., Fu G., Guo L. Vasodilatation produced by fasudil mesylate in vivo and in vitro. Vascul. Pharmacol. 2011;55:121–126. doi: 10.1016/j.vph.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 190.Jayakumar T., Sheu J.-R. Cardiovascular pharmacological actions of rutaecarpine, a quinazolinocarboline alkaloid isolated from Evodia rutaecarpa. J. Exp. Clin. Med. 2011;3:63–69. doi: 10.1016/j.jecm.2011.02.004. [DOI] [Google Scholar]
- 191.Choi H., Tostes R.C., Webb R.C. Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J. Am. Soc. Hypertens. 2011;5:154–160. doi: 10.1016/j.jash.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Medei E., Lima-Leopoldo A.P., Pereira-Junior P.P., Leopoldo A.S., Campos D.H.S., Raimundo J.M., Sudo R.T., Zapata-Sudo G., Bruder-Nascimento T., Cordellini S. Could a high-fat diet rich in unsaturated fatty acids impair the cardiovascular system? Can. J. Cardiol. 2010;26:542–548. doi: 10.1016/S0828-282X(10)70469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang J., Dong M.Q., Liu M.L., Xu D.Q., Luo Y., Zhang B., Liu L.L., Xu M., Zhao P.T., Gao Y.Q., et al. Tanshinone IIA modulates pulmonary vascular response to agonist and hypoxia primarily via inhibiting Ca2+ influx and release in normal and hypoxic pulmonary hypertension rats. Eur. J. Pharmacol. 2010;640:129–138. doi: 10.1016/j.ejphar.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 194.Shen D., Xu X., Zhang L., Wu H., Peng L. Identification of a nitric oxide-dependent hypotensive effect of anticoagulation factor II from the venom of Agkistrodon acutus. Biochem. Pharmacol. 2010;79:498–506. doi: 10.1016/j.bcp.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 195.Chen G.P., Li L., Yang Y., Fu M., Yao L., Wu T., Zhang X.Q., Hu S.J. Chronic inhibition of farnesyl pyrophosphate synthase improves endothelial function in spontaneously hypertensive rats. Biochem. Pharmacol. 2010;80:1684–1689. doi: 10.1016/j.bcp.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 196.Romero M., Jimenez R., Hurtado B., Moreno J.M., Rodriguez-Gomez I., Lopez-Sepulveda R., Zarzuelo A., Perez-Vizcaino F., Tamargo J., Vargas F., et al. Lack of beneficial metabolic effects of quercetin in adult spontaneously hypertensive rats. Eur. J. Pharmacol. 2010;627:242–250. doi: 10.1016/j.ejphar.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 197.Subramani J., Leo M.D., Kathirvel K., Arunadevi R., Singh T.U., Prakash V.R., Mishra S.K. Essential role of nitric oxide in sepsis-induced impairment of endothelium-derived hyperpolarizing factor-mediated relaxation in rat pulmonary artery. Eur. J. Pharmacol. 2010;630:84–91. doi: 10.1016/j.ejphar.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 198.Zhang H.T., Wang Y., Deng X.L., Dong M.Q., Zhao L.M., Wang Y.W. Daidzein relaxes rat cerebral basilar artery via activation of large-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Eur. J. Pharmacol. 2010;630:100–106. doi: 10.1016/j.ejphar.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 199.Singh T.U., Kathirvel K., Choudhury S., Garg S.K., Mishra S.K. Eicosapentaenoic acid-induced endothelium-dependent and -independent relaxation of sheep pulmonary artery. Eur. J. Pharmacol. 2010;636:108–113. doi: 10.1016/j.ejphar.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 200.Enkhjargal B., Hashimoto M., Sakai Y., Shido O. Characterization of vasoconstrictor-induced relaxation in the cerebral basilar artery. Eur. J. Pharmacol. 2010;637:118–123. doi: 10.1016/j.ejphar.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 201.Sanchez A., Recio P., Orensanz L.M., Bustamante S., Navarro-Dorado J., Climent B., Benedito S., Garcia-Sacristan A., Prieto D., Hernandez M. Mechanisms involved in the effects of endothelin-1 in pig prostatic small arteries. Eur. J. Pharmacol. 2010;640:190–196. doi: 10.1016/j.ejphar.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 202.Bayram Z., Golbasi I., Ozdem S.S. The role of nitric oxide and potassium channels in the effect of adrenomedullin in human internal thoracic arteries. Regul. Pept. 2010;161:92–96. doi: 10.1016/j.regpep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 203.Franca-Silva M.S., Luciano M.N., Ribeiro T.P., Silva J.S., Santos A.F., Franca K.C., Nakao L.S., Athayde-Filho P.F., Braga V.A., Medeiros I.A. The 2-nitrate-1,3-dibuthoxypropan, a new nitric oxide donor, induces vasorelaxation in mesenteric arteries of the rat. Eur. J. Pharmacol. 2012;690:170–175. doi: 10.1016/j.ejphar.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 204.Perusquia M., Espinoza J., Montano L.M., Stallone J.N. Regional differences in the vasorelaxing effects of testosterone and its 5-reduced metabolites in the canine vasculature. Vascul. Pharmacol. 2012;56:176–182. doi: 10.1016/j.vph.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Arai H., Zaima K., Mitsuta E., Tamamoto H., Saito A., Hirasawa Y., Rahman A., Kusumawati I., Zaini N.C., Morita H. Alstiphyllanines I–O, ajmaline type alkaloids from Alstonia macrophylla showing vasorelaxant activity. Bioorg. Med. Chem. 2012;20:3454–3459. doi: 10.1016/j.bmc.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 206.Marrachelli V.G., Centeno J.M., Miranda I., Castello-Ruiz M., Burguete M.C., Jover-Mengual T., Salom J.B., Torregrosa G., Miranda F.J., Alborch E. Diabetes impairs the atrial natriuretic peptide relaxant action mediated by potassium channels and prostacyclin in the rabbit renal artery. Pharmacol. Res. 2012;66:392–400. doi: 10.1016/j.phrs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 207.Lu Y., Fu Y., Ge Y., Juncos L.A., Reckelhoff J.F., Liu R. The vasodilatory effect of testosterone on renal afferent arterioles. Gend. Med. 2012;9:103–111. doi: 10.1016/j.genm.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sodhi K., Puri N., Inoue K., Falck J.R., Schwartzman M.L., Abraham N.G. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prostaglandins Other Lipid Mediat. 2012;98:133–142. doi: 10.1016/j.prostaglandins.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lesniewski L.A., Zigler M.C., Durrant J.R., Donato A.J., Seals D.R. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech. Ageing Dev. 2012;133:368–371. doi: 10.1016/j.mad.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shuto H., Tominaga K., Yamauchi A., Ikeda M., Kusaba K., Mitsunaga D., Hirabara Y., Egawa T., Takano Y., Kataoka Y. The statins fluvastatin and pravastatin exert anti-flushing effects by improving vasomotor dysfunction through nitric oxide-mediated mechanisms in ovariectomized animals. Eur. J. Pharmacol. 2011;651:234–239. doi: 10.1016/j.ejphar.2010.10.084. [DOI] [PubMed] [Google Scholar]
- 211.Toba H., Morishita M., Tojo C., Nakano A., Oshima Y., Kojima Y., Yoshida M., Nakashima K., Wang J., Kobara M., et al. Recombinant human erythropoietin ameliorated endothelial dysfunction and macrophage infiltration by increasing nitric oxide in hypertensive 5/6 nephrectomized rat aorta. Eur. J. Pharmacol. 2011;656:81–87. doi: 10.1016/j.ejphar.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 212.Garcia-Villalon A.L., Fernandez N., Monge L., Dieguez G. Coronary response to diadenosine tetraphosphate after ischemia-reperfusion in the isolated rat heart. Eur. J. Pharmacol. 2011;660:394–401. doi: 10.1016/j.ejphar.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 213.Medeiros M.A., Pinho J.F., De-Lira D.P., Barbosa-Filho J.M., Araujo D.A., Cortes S.F., Lemos V.S., Cruz J.S. Curine, a bisbenzylisoquinoline alkaloid, blocks l-type Ca2+ channels and decreases intracellular Ca2+ transients in A7r5 cells. Eur. J. Pharmacol. 2011;669:100–107. doi: 10.1016/j.ejphar.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 214.Wang X.B., Jin H.F., Tang C.S., Du J.B. The biological effect of endogenous sulfur dioxide in the cardiovascular system. Eur. J. Pharmacol. 2011;670:1–6. doi: 10.1016/j.ejphar.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 215.de Buys Roessingh A., Fouquet V., Aigrain Y., Mercier J.-C., de Lagausie P., Dinh-Xuan A.T. Nitric oxide activity through guanylate cyclase and phosphodiesterase modulation is impaired in fetal lambs with congenital diaphragmatic hernia. J. Pediatr. Surg. 2011;46:1516–1522. doi: 10.1016/j.jpedsurg.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 216.Takir S., Uydes-Dogan B.S., Ozdemir O. Retina evokes biphasic relaxations in retinal artery unrelated to endothelium, K(V), K(ATP), K(Ca) channels and methyl palmitate. Microvasc. Res. 2011;81:295–302. doi: 10.1016/j.mvr.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 217.Dasiewicz P.J., Conlon J.M., Anderson W.G. Cardiovascular and vasoconstrictive actions of skate bradykinin in the little skate, Leucoraja erinacea (Elasmobranchii) Gen. Comp. Endocrinol. 2011;174:89–96. doi: 10.1016/j.ygcen.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 218.Fang Y., Nicol L., Harouki N., Monteil C., Wecker D., Debunne M., Bauer F., Lallemand F., Richard V., Thuillez C., et al. Improvement of left ventricular diastolic function induced by β-blockade: A comparison between nebivolol and metoprolol. J. Mol. Cell. Cardiol. 2011;51:168–176. doi: 10.1016/j.yjmcc.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 219.Wong W.T., Ng C.H., Tsang S.Y., Huang Y., Chen Z.Y. Relative contribution of individual oxidized components in ox-LDL to inhibition on endothelium-dependent relaxation in rat aorta. Nutr. Metab. Cardiovasc. Dis. 2011;21:157–164. doi: 10.1016/j.numecd.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 220.Chakkarwar V.A. Fenofibrate attenuates nicotine-induced vascular endothelial dysfunction in the rat. Vascul. Pharmacol. 2011;55:163–168. doi: 10.1016/j.vph.2011.08.215. [DOI] [PubMed] [Google Scholar]
- 221.Bertinaria M., Guglielmo S., Rolando B., Giorgis M., Aragno C., Fruttero R., Gasco A., Parapini S., Taramelli D., Martins Y.C., et al. Amodiaquine analogues containing NO-donor substructures: synthesis and their preliminary evaluation as potential tools in the treatment of cerebral malaria. Eur. J. Med. Chem. 2011;46:1757–1767. doi: 10.1016/j.ejmech.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 222.Parlar A., Can C., Erol A., Ulker S. Posttransplantation therapeutic rapamycin concentration protects nitric oxide-related vascular endothelial function: Comparative effects in rat thoracic aorta and coronary endothelial cell culture. Transpl. Proc. 2010;42:1923–1930. doi: 10.1016/j.transproceed.2010.03.134. [DOI] [PubMed] [Google Scholar]
- 223.Lin Y.L., Dai Z.K., Lin R.J., Chu K.S., Chen I.J., Wu J.R., Wu B.N. Baicalin, a flavonoid from Scutellaria baicalensis Georgi, activates large-conductance Ca2+-activated K+ channels via cyclic nucleotide-dependent protein kinases in mesenteric artery. Phytomedicine. 2010;17:760–770. doi: 10.1016/j.phymed.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 224.Marrachelli V.G., Miranda F.J., Centeno J.M., Salom J.B., Torregrosa G., Jover-Mengual T., Perez A.M., Moro M.A., Alborch E. Role of NO-synthases and cyclooxygenases in the hyperreactivity of male rabbit carotid artery to testosterone under experimental diabetes. Pharmacol. Res. 2010;61:62–70. doi: 10.1016/j.phrs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 225.Novella S., Dantas A.P., Segarra G., Novensa L., Bueno C., Heras M., Hermenegildo C., Medina P. Gathering of aging and estrogen withdrawal in vascular dysfunction of senescent accelerated mice. Exp. Gerontol. 2010;45:868–874. doi: 10.1016/j.exger.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 226.Raffetto J.D., Qiao X., Beauregard K.G., Khalil R.A. Estrogen receptor-mediated enhancement of venous relaxation in female rat: implications in sex-related differences in varicose veins. J. Vasc. Surg. 2010;51:972–981. doi: 10.1016/j.jvs.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Khoo N.K., White C.R., Pozzo-Miller L., Zhou F., Constance C., Inoue T., Patel R.P., Parks D.A. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic. Biol. Med. 2010;49:339–347. doi: 10.1016/j.freeradbiomed.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Deng J., Zhao R., Zhang Z., Wang J. Changes in vasoreactivity of rat large- and medium-sized arteries induced by hyperthyroidism. Exp. Toxicol. Pathol. 2010;62:317–322. doi: 10.1016/j.etp.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 229.Seto S.W., Lam T.Y., Or P.M., Lee W.Y., Au A.L., Poon C.C., Li R.W., Chan S.W., Yeung J.H., Leung G.P., et al. Folic acid consumption reduces resistin level and restores blunted acetylcholine-induced aortic relaxation in obese/diabetic mice. J. Nutr. Biochem. 2010;21:872–880. doi: 10.1016/j.jnutbio.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 230.Lolli M.L., Rolando B., Tosco P., Chaurasia S., di Stilo A., Lazzarato L., Gorassini E., Ferracini R., Oliaro-Bosso S., Fruttero R., et al. Synthesis and preliminary pharmacological characterisation of a new class of nitrogen-containing bisphosphonates (N-BPs) Bioorg. Med. Chem. 2010;18:2428–2438. doi: 10.1016/j.bmc.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 231.Marrachelli V.G., Miranda F.J., Centeno J.M., Burguete M.C., Castello-Ruiz M., Jover-Mengual T., Perez A.M., Salom J.B., Torregrosa G., Alborch E. Mechanisms involved in the relaxant action of testosterone in the renal artery from male normoglycemic and diabetic rabbits. Pharmacol. Res. 2010;61:149–156. doi: 10.1016/j.phrs.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 232.Matsumoto T., Ishida K., Nakayama N., Taguchi K., Kobayashi T., Kamata K. Mechanisms underlying the losartan treatment-induced improvement in the endothelial dysfunction seen in mesenteric arteries from type 2 diabetic rats. Pharmacol. Res. 2010;62:271–281. doi: 10.1016/j.phrs.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 233.Olukman M., Orhan C.E., Celenk F.G., Ulker S. Apocynin restores endothelial dysfunction in streptozotocin diabetic rats through regulation of nitric oxide synthase and NADPH oxidase expressions. J. Diabetes Complicat. 2010;24:415–423. doi: 10.1016/j.jdiacomp.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 234.Yang H.H., van Breemen C., Chung A.W. Vasomotor dysfunction in the thoracic aorta of Marfan syndrome is associated with accumulation of oxidative stress. Vascul. Pharmacol. 2010;52:37–45. doi: 10.1016/j.vph.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 235.Liao W.C., Hou M.C., Wang G.J., Yu K.W., Lee F.Y., Lin H.C., Lee S.D. Sepsis worsening vascular hyporeactivity of the superior mesenteric artery in portal vein-ligated rats. J. Chin. Med. Assoc. 2010;73:462–470. doi: 10.1016/S1726-4901(10)70100-3. [DOI] [PubMed] [Google Scholar]
- 236.Dominguez J.M., 2nd, Prisby R.D., Muller-Delp J.M., Allen M.R., Delp M.D. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone. 2010;46:813–819. doi: 10.1016/j.bone.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li Y.H., Xu Q., Xu W.H., Guo X.H., Zhang S., Chen Y.D. Mechanisms of protection against diabetes-induced impairment of endothelium-dependent vasorelaxation by Tanshinone IIA. Biochim. Biophys. Acta. 2015;1850:813–823. doi: 10.1016/j.bbagen.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 238.de Candia M., Marini E., Zaetta G., Cellamare S., di Stilo A., Altomare C.D. New organic nitrate-containing benzyloxy isonipecotanilide derivatives with vasodilatory and anti-platelet activity. Eur. J. Pharm. Sci. 2015;72:69–80. doi: 10.1016/j.ejps.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 239.Yoon J., Park M., hyung Lee J., Min B.S., Ryoo S. Endothelial nitric oxide synthase activation through obacunone-dependent arginase inhibition restored impaired endothelial function in ApoE-null mice. Vascul. Pharmacol. 2014;60:102–109. doi: 10.1016/j.vph.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 240.Kagota S., Maruyama K., Wakuda H., McGuire J.J., Yoshikawa N., Nakamura K., Shinozuka K. Disturbance of vasodilation via protease-activated receptor 2 in SHRSP.Z-Lepr fa/IzmDmcr rats with metabolic syndrome. Vascul. Pharmacol. 2014;63:46–54. doi: 10.1016/j.vph.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 241.Kline L.W., Karpinski E. The flavonoid chrysin, an endocrine disrupter, relaxes cholecystokinin- and KCl-induced tension in male guinea pig gallbladder strips through multiple signaling pathways. Steroids. 2014;79:64–69. doi: 10.1016/j.steroids.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 242.Salomone S., Foresti R., Villari A., Giurdanella G., Drago F., Bucolo C. Regulation of vascular tone in rabbit ophthalmic artery: Cross talk of endogenous and exogenous gas mediators. Biochem. Pharmacol. 2014;92:661–668. doi: 10.1016/j.bcp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 243.Liu L.L., Yan L., Chen Y.H., Zeng G.H., Zhou Y., Chen H.P., Peng W.J., He M., Huang Q.R. A role for diallyl trisulfide in mitochondrial antioxidative stress contributes to its protective effects against vascular endothelial impairment. Eur. J. Pharmacol. 2014;725:23–31. doi: 10.1016/j.ejphar.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 244.Matsumoto T., Watanabe S., Kawamura R., Taguchi K., Kobayashi T. Epigallocatechin gallate attenuates ET-1-induced contraction in carotid artery from type 2 diabetic OLETF rat at chronic stage of disease. Life Sci. 2014;118:200–205. doi: 10.1016/j.lfs.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 245.Schrammel A., Mussbacher M., Wolkart G., Stessel H., Pail K., Winkler S., Schweiger M., Haemmerle G., Al Zoughbi W., Hofler G., et al. Endothelial dysfunction in adipose triglyceride lipase deficiency. Biochim. Biophys. Acta. 2014;1841:906–917. doi: 10.1016/j.bbalip.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kivimäki A.S., Siltari A., Ehlers P.I., Korpela R., Vapaatalo H. Lingonberry juice negates the effects of a high salt diet on vascular function and low-grade inflammation. J. Funct. Foods. 2014;7:238–245. doi: 10.1016/j.jff.2014.02.005. [DOI] [Google Scholar]
- 247.de Sá L.Z.M., Castro P.F., Lino F.M., Bernardes M.J., Viegas J.C., Dinis T.C., Santana M.J., Romao W., Vaz B.G., Lião L.M. Antioxidant potential and vasodilatory activity of fermented beverages of jabuticaba berry (Myrciaria jaboticaba) J. Funct. Foods. 2014;8:169–179. doi: 10.1016/j.jff.2014.03.009. [DOI] [Google Scholar]
- 248.Zhou Z., de Wijs-Meijler D., Lankhuizen I., Jankowski J., Jankowski V., Jan Danser A.H., Duncker D.J., Merkus D. Blunted coronary vasodilator response to uridine adenosine tetraphosphate in post-infarct remodeled myocardium is due to reduced P1 receptor activation. Pharmacol. Res. 2013;77:22–29. doi: 10.1016/j.phrs.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 249.Gadkari T.V., Cortes N., Madrasi K., Tsoukias N.M., Joshi M.S. Agmatine induced NO dependent rat mesenteric artery relaxation and its impairment in salt-sensitive hypertension. Nitric Oxide. 2013;35:65–71. doi: 10.1016/j.niox.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mamo Y.A., Angus J.A., Ziogas J., Soeding P.F., Wright C.E. The role of voltage-operated and non-voltage-operated calcium channels in endothelin-induced vasoconstriction of rat cerebral arteries. Eur. J. Pharmacol. 2014;742:65–73. doi: 10.1016/j.ejphar.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 251.Hedegaard E.R., Nielsen B.D., Mogensen S., Rembold C.M., Frobert O., Simonsen U. Mechanisms involved in increased sensitivity to adenosine A(2A) receptor activation and hypoxia-induced vasodilatation in porcine coronary arteries. Eur. J. Pharmacol. 2014;723:216–226. doi: 10.1016/j.ejphar.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 252.Chaothanaphat N., Dhumma-Upakorn P., Jianmongkol S. In vitro modulating effects of glutathione on vascular tension and involvement of extracellular calcium. Drug Discov. Ther. 2010;4:19–25. [PubMed] [Google Scholar]
- 253.Rodrigues G.J., Cicillini S.A., Silva R.S., Bendhack L.M. Mechanisms underlying the vascular relaxation induced by a new nitric oxide generator. Nitric Oxide. 2011;25:331–337. doi: 10.1016/j.niox.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 254.Vlasova M.A., Tarasova O.S., Riikonen J., Raula J., Lobach A.S., Borzykh A.A., Smirin B.V., Kauppinen E.I., Eletskii A.V., Herzig K.H., et al. Injected nanoparticles: the combination of experimental systems to assess cardiovascular adverse effects. Eur. J. Pharm. Biopharm. 2014;87:64–72. doi: 10.1016/j.ejpb.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 255.Laspas P., Goloborodko E., Sniatecki J.J., Kordasz M.L., Manicam C., Wojnowski L., Li H., Patzak A., Pfeiffer N., Gericke A. Role of nitric oxide synthase isoforms for ophthalmic artery reactivity in mice. Exp. Eye Res. 2014;127:1–8. doi: 10.1016/j.exer.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 256.Sakakibara K., Feng G.G., Li J., Akahori T., Yasuda Y., Nakamura E., Hatakeyama N., Fujiwara Y., Kinoshita H. Kynurenine causes vasodilation and hypotension induced by activation of KCNQ-encoded voltage-dependent K+ channels. J. Pharmacol. Sci. 2015;129:31–37. doi: 10.1016/j.jphs.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 257.Zarzuelo M.J., Lopez-Sepulveda R., Sanchez M., Romero M., Gomez-Guzman M., Ungvary Z., Perez-Vizcaino F., Jimenez R., Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: Implications for vascular aging. Biochem. Pharmacol. 2013;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 258.Kurtel H., Rodrigues S.F., Yilmaz C.E., Yildirim A., Granger D.N. Impaired vasomotor function induced by the combination of hypertension and hypercholesterolemia. J. Am. Soc. Hypertens. 2013;7:14–23. doi: 10.1016/j.jash.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhao J., Suyama A., Tanaka M., Matsui T. Ferulic acid enhances the vasorelaxant effect of epigallocatechin gallate in tumor necrosis factor-α-induced inflammatory rat aorta. J. Nutr. Biochem. 2014;25:807–814. doi: 10.1016/j.jnutbio.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 260.Kumar A., Kumar A., Jaggi A.S., Singh N. Efficacy of Cilostazol a selective phosphodiesterase-3 inhibitor in rat model of Streptozotocin diabetes induced vascular dementia. Pharmacol. Biochem. Behav. 2015;135:20–30. doi: 10.1016/j.pbb.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 261.Ying Z., Xie X., Chen M., Yi K., Rajagopalan S. α-lipoic acid activates eNOS through activation of PI3-kinase/Akt signaling pathway. Vascul. Pharmacol. 2015;64:28–35. doi: 10.1016/j.vph.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Soloviev A., Zholos A., Ivanova I., Novokhatska T., Tishkin S., Raevska A., Stroyuk A., Yefanov V. Plasmonic gold nanoparticles possess the ability to open potassium channels in rat thoracic aorta smooth muscles in a remote control manner. Vascul. Pharmacol. 2015;72:190–196. doi: 10.1016/j.vph.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 263.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Moncada S., Korbut R., Bunting S., Vane J.R. Prostacyclin is a circulating hormone. Nature. 1978;273:767–768. doi: 10.1038/273767a0. [DOI] [PubMed] [Google Scholar]
- 265.Palmer R.M., Ferrige A.G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 266.Wang R. Hydrogen sulfide: A new EDRF. Kidney Int. 2009;76:700–704. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- 267.Quillon A., Fromy B., Debret R. Endothelium microenvironment sensing leading to nitric oxide mediated vasodilation: A review of nervous and biomechanical signals. Nitric Oxide. 2015;45:20–26. doi: 10.1016/j.niox.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 268.Vitecek J., Lojek A., Valacchi G., Kubala L. Arginine-based inhibitors of nitric oxide synthase: therapeutic potential and challenges. Mediators Inflamm. 2012;2012:318087. doi: 10.1155/2012/318087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Furfine E.S., Harmon M.F., Paith J.E., Knowles R.G., Salter M., Kiff R.J., Duffy C., Hazelwood R., Oplinger J.A., Garvey E.P. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-l-thiocitrulline and S-ethyl-l-thiocitrulline. J. Biol. Chem. 1994;269:26677–26683. [PubMed] [Google Scholar]
- 270.Klatt P., Schmidt K., Brunner F., Mayer B. Inhibitors of brain nitric oxide synthase. Binding kinetics, metabolism, and enzyme inactivation. J. Biol. Chem. 1994;269:1674–1680. [PubMed] [Google Scholar]
- 271.Balligand J.L., Kelly R.A., Marsden P.A., Smith T.W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc. Natl. Acad. Sci. USA. 1993;90:347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kubes P., Suzuki M., Granger D.N. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pfeiffer S., Leopold E., Schmidt K., Brunner F., Mayer B. Inhibition of nitric oxide synthesis by NG-nitro-l-arginine methyl ester (l-NAME): Requirement for bioactivation to the free acid, NG-nitro-l-arginine. Br. J. Pharmacol. 1996;118:1433–1440. doi: 10.1111/j.1476-5381.1996.tb15557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Page C.P., Curtis M.J., Sutter M. Farmacologia Integrada. Elsevier Espanha; Madrid, Spain: 1998. [Google Scholar]
- 275.Suleyman H., Demircan B., Karagoz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007;59:247–258. [PubMed] [Google Scholar]
- 276.Riendeau D., Percival M.D., Boyce S., Brideau C., Charleson S., Cromlish W., Ethier D., Evans J., Falgueyret J.P., Ford-Hutchinson A.W., et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br. J. Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dannhardt G., Kiefer W. Cyclooxygenase inhibitors—Current status and future prospects. Eur. J. Med. Chem. 2001;36:109–126. doi: 10.1016/S0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- 278.Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 279.Mayer B., Brunner F., Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem. Pharmacol. 1993;45:367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- 280.Kontos H.A., Wei E.P. Hydroxyl radical-dependent inactivation of guanylate cyclase in cerebral arterioles by methylene blue and by LY83583. Stroke. 1993;24:427–434. doi: 10.1161/01.STR.24.3.427. [DOI] [PubMed] [Google Scholar]
- 281.Olson L.J., Knych E.T., Jr., Herzig T.C., Drewett J.G. Selective guanylyl cyclase inhibitor reverses nitric oxide-induced vasorelaxation. Hypertension. 1997;29:254–261. doi: 10.1161/01.HYP.29.1.254. [DOI] [PubMed] [Google Scholar]
- 282.Moro M.A., Russel R.J., Cellek S., Lizasoain I., Su Y., Darley-Usmar V.M., Radomski M.W., Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc. Natl. Acad. Sci. USA. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Schrammel A., Behrends S., Schmidt K., Koesling D., Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- 284.Valtcheva N., Nestorov P., Beck A., Russwurm M., Hillenbrand M., Weinmeister P., Feil R. The commonly used cGMP-dependent protein kinase type I (cGKI) inhibitor Rp-8-Br-PET-cGMPS can activate cGKI in vitro and in intact cells. J. Biol. Chem. 2009;284:556–562. doi: 10.1074/jbc.M806161200. [DOI] [PubMed] [Google Scholar]
- 285.Baltoumas F.A., Theodoropoulou M.C., Hamodrakas S.J. Interactions of the α-subunits of heterotrimeric G-proteins with GPCRs, effectors and RGS proteins: A critical review and analysis of interacting surfaces, conformational shifts, structural diversity and electrostatic potentials. J. Struct. Biol. 2013;182:209–218. doi: 10.1016/j.jsb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 286.Weir M.R., Dzau V.J. The renin-angiotensin-aldosterone system: A specific target for hypertension management. Am. J. Hypertens. 1999;12:205S–213S. doi: 10.1016/S0895-7061(99)00103-X. [DOI] [PubMed] [Google Scholar]
- 287.de Gasparo M., Catt K.J., Inagami T., Wright J.W., Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 288.Jakala P., Pere E., Lehtinen R., Turpeinen A., Korpela R., Vapaatalo H. Cardiovascular activity of milk casein-derived tripeptides and plant sterols in spontaneously hypertensive rats. J. Physiol. Pharmacol. 2009;60:11–20. [PubMed] [Google Scholar]
- 289.Chen J., Yildiz O., Purdy R.E. Phenylephrine precontraction increases the sensitivity of rabbit femoral artery to serotonin by enabling 5-HT1-like receptors. J. Cardiovasc. Pharmacol. 2000;35:863–870. doi: 10.1097/00005344-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 290.Bockaert J., Claeysen S., Bécamel C., Dumuis A., Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 291.Schoeffter P., Hoyer D. 5-Hydroxytryptamine (5-HT)-induced endothelium-dependent relaxation of pig coronary arteries is mediated by 5-HT receptors similar to the 5-HT1D receptor subtype. J. Pharmacol. Exp. Ther. 1990;252:387–395. [PubMed] [Google Scholar]
- 292.Yildiz O., Cicek S., Ay I., Demirkilic U., Tuncer M. Hypertension increases the contractions to sumatriptan in the human internal mammary artery. Ann. Thorac. Surg. 1996;62:1392–1395; discussion 1396. doi: 10.1016/0003-4975(96)00674-1. [DOI] [PubMed] [Google Scholar]
- 293.Kuhr F., Lowry J., Zhang Y., Brovkovych V., Skidgel R.A. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides. 2010;44:145–154. doi: 10.1016/j.npep.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Leeb-Lundberg L.M., Marceau F., Muller-Esterl W., Pettibone D.J., Zuraw B.L. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 295.Davenport A.P., O′Reilly G., Molenaar P., Maguire J.J., Kuc R.E., Sharkey A., Bacon C.R., Ferro A. Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: Evidence for expression of ETB receptors in human vascular smooth muscle. J. Cardiovasc. Pharmacol. 1993;22:S22–S25. doi: 10.1097/00005344-199322008-00008. [DOI] [PubMed] [Google Scholar]
- 296.Levin E.R. Endothelins. N. Engl. J. Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 297.Mazzuca M.Q., Khalil R.A. Vascular endothelin receptor type B: Structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 2012;84:147–162. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ogawa Y., Nakao K., Arai H., Nakagawa O., Hosoda K., Suga S., Nakanishi S., Imura H. Molecular cloning of a non-isopeptide-selective human endothelin receptor. Biochem. Biophys. Res. Commun. 1991;178:248–255. doi: 10.1016/0006-291X(91)91806-N. [DOI] [PubMed] [Google Scholar]
- 299.Seo B., Oemar B.S., Siebenmann R., Von Segesser L., Lüscher T. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89:1203–1208. doi: 10.1161/01.CIR.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 300.Caulfield M.P., Birdsall N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 301.Chabner B., Brunton L., Knollman B. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 20th ed. McGraw-Hill Education; New York, NY, USA: 2011. [Google Scholar]
- 302.Ishii M., Kurachi Y. Muscarinic acetylcholine receptors. Curr. Pharm. Des. 2006;12:3573–3581. doi: 10.2174/138161206778522056. [DOI] [PubMed] [Google Scholar]
- 303.Benoist C.C., Wright J.W., Zhu M., Appleyard S.M., Wayman G.A., Harding J.W. Facilitation of hippocampal synaptogenesis and spatial memory by C-terminal truncated Nle1-angiotensin IV analogs. J. Pharmacol. Exp. Ther. 2011;339:35–44. doi: 10.1124/jpet.111.182220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Catt K.J., Mendelsohn F.A., Millan M.A., Aguilera G. The role of angiotensin II receptors in vascular regulation. J. Cardiovasc. Pharmacol. 1984;6(Suppl. 4):S575–S586. doi: 10.1097/00005344-198406004-00004. [DOI] [PubMed] [Google Scholar]
- 305.D’Amore A., Black M.J., Thomas W.G. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension. 2005;46:1347–1354. doi: 10.1161/01.HYP.0000193504.51489.cf. [DOI] [PubMed] [Google Scholar]
- 306.Wright J.W., Harding J.W. Brain renin-angiotensin—A new look at an old system. Prog. Neurobiol. 2011;95:49–67. doi: 10.1016/j.pneurobio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 307.Wagner O.F., Christ G., Wojta J., Vierhapper H., Parzer S., Nowotny P.J., Schneider B., Waldhausl W., Binder B.R. Polar secretion of endothelin-1 by cultured endothelial cells. J. Biol. Chem. 1992;267:16066–16068. [PubMed] [Google Scholar]
- 308.Cocks T.M., Angus J.A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- 309.He G.W., Yang C.Q. Effect of thromboxane A2 antagonist GR32191B on prostanoid and nonprostanoid receptors in the human internal mammary artery. J. Cardiovasc. Pharmacol. 1995;26:13–19. doi: 10.1097/00005344-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 310.Leemhuis J., Boutillier S., Schmidt G., Meyer D.K. The protein kinase A inhibitor H89 acts on cell morphology by inhibiting Rho kinase. J. Pharmacol. Exp. Ther. 2002;300:1000–1007. doi: 10.1124/jpet.300.3.1000. [DOI] [PubMed] [Google Scholar]
- 311.Petersen R.K., Madsen L., Pedersen L.M., Hallenborg P., Hagland H., Viste K., Doskeland S.O., Kristiansen K. Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol. Cell. Biol. 2008;28:3804–3816. doi: 10.1128/MCB.00709-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Emery A.C., Eiden M.V., Eiden L.E. A new site and mechanism of action for the widely used adenylate cyclase inhibitor SQ22,536. Mol. Pharmacol. 2013;83:95–105. doi: 10.1124/mol.112.081760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bouschet T., Perez V., Fernandez C., Bockaert J., Eychene A., Journot L. Stimulation of the ERK pathway by GTP-loaded Rap1 requires the concomitant activation of Ras, protein kinase C, and protein kinase A in neuronal cells. J. Biol. Chem. 2003;278:4778–4785. doi: 10.1074/jbc.M204652200. [DOI] [PubMed] [Google Scholar]
- 314.Yuan W., Bers D.M. Protein kinase inhibitor H-89 reverses forskolin stimulation of cardiac l-type calcium current. Am. J. Physiol. 1995;268:C651–C659. doi: 10.1152/ajpcell.1995.268.3.C651. [DOI] [PubMed] [Google Scholar]
- 315.Satake N., Fujimoto S., Shibata S. The potentiation of nitroglycerin-induced relaxation by PKG inhibition in rat aortic rings. Gen. Pharmacol. 1996;27:701–705. doi: 10.1016/0306-3623(95)00116-6. [DOI] [PubMed] [Google Scholar]
- 316.Buch J.G. Clinically Oriented Pharmacology. PDU Medical College; Rajkot, Gujarat, India: 2010. Version 2. [Google Scholar]
- 317.Walch L., Gascard J.P., Dulmet E., Brink C., Norel X. Evidence for a M1 muscarinic receptor on the endothelium of human pulmonary veins. Br. J. Pharmacol. 2000;130:73–78. doi: 10.1038/sj.bjp.0703301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Bhattacharjee A.K., Pomponio J.W., Evans S.A., Pervitsky D., Gordon R.K. Discovery of subtype selective muscarinic receptor antagonists as alternatives to atropine using in silico pharmacophore modeling and virtual screening methods. Bioorg. Med. Chem. 2013;21:2651–2662. doi: 10.1016/j.bmc.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 319.Zholos A.V., Bolton T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Archer S.L., Rusch N.J. Potassium Channels in Cardiovascular Biology. Springer; New York, NY, USA: 2001. [Google Scholar]
- 321.Burnham M.P., Bychkov R., Feletou M., Richards G.R., Vanhoutte P.M., Weston A.H., Edwards G. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: Relevance to EDHF. Br. J. Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Bychkov R., Burnham M.P., Richards G.R., Edwards G., Weston A.H., Feletou M., Vanhoutte P.M. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br. J. Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Feletou M., Vanhoutte P. EDHF: The Complete Story. CRC Press; Hoboken, NJ, USA: 2005. [Google Scholar]
- 324.Frieden M., Sollini M., Beny J. Substance P and bradykinin activate different types of KCa currents to hyperpolarize cultured porcine coronary artery endothelial cells. J. Physiol. 1999;519:361–371. doi: 10.1111/j.1469-7793.1999.0361m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Marchenko S.M., Sage S.O. Calcium-activated potassium channels in the endothelium of intact rat aorta. J. Physiol. 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Taylor M.S., Bonev A.D., Gross T.P., Eckman D.M., Brayden J.E., Bond C.T., Adelman J.P., Nelson M.T. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ. Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 327.Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J. Gen. Physiol. 1967;50:1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Schumacher M.A., Rivard A.F., Bachinger H.P., Adelman J.P. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 329.Boyd A.E., 3rd, Aguilar-Bryan L., Nelson D.A. Molecular mechanisms of action of glyburide on the β cell. Am. J. Med. 1990;89:3S–10S. doi: 10.1016/0002-9343(90)90330-G. [DOI] [PubMed] [Google Scholar]
- 330.Standen N.B., Quayle J.M., Davies N.W., Brayden J.E., Huang Y., Nelson M.T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 331.Katsuda Y., Egashira K., Ueno H., Akatsuka Y., Narishige T., Arai Y., Takayanagi T., Shimokawa H., Takeshita A. Glibenclamide, a selective inhibitor of ATP-sensitive K+ channels, attenuates metabolic coronary vasodilatation induced by pacing tachycardia in dogs. Circulation. 1995;92:511–517. doi: 10.1161/01.CIR.92.3.511. [DOI] [PubMed] [Google Scholar]
- 332.Chandy K.G., Wulff H., Beeton C., Pennington M., Gutman G.A., Cahalan M.D. K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S.H., Tanasa B., Hogan P.G., Lewis R.S., Daly M., Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 334.Wellman G.C., Bevan J.A. Barium inhibits the endothelium-dependent component of flow but not acetylcholine-induced relaxation in isolated rabbit cerebral arteries. J. Pharmacol. Exp. Ther. 1995;274:47–53. [PubMed] [Google Scholar]
- 335.Golan D.E., Tashjian A.H., Armstrong E.J. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Wolters Kluwer Health; Philadelphia, PA, USA: 2011. [Google Scholar]
- 336.Furberg C.D., Psaty B.M., Meyer J.V. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326–1331. doi: 10.1161/01.CIR.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 337.Ma H.T., Venkatachalam K., Parys J.B., Gill D.L. Modification of store-operated channel coupling and inositol trisphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J. Biol. Chem. 2002;277:6915–6922. doi: 10.1074/jbc.M107755200. [DOI] [PubMed] [Google Scholar]
- 338.Prakriya M., Lewis R.S. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J. Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Bootman M.D., Collins T.J., MacKenzie L., Roderick H.L., Berridge M.J., Peppiatt C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 340.Adding L.C., Bannenberg G.L., Gustafsson L.E. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardiovasc. Drug Rev. 2001;19:41–56. doi: 10.1111/j.1527-3466.2001.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 341.Fellner S.K., Arendshorst W.J. Store-operated Ca2+ entry is exaggerated in fresh preglomerular vascular smooth muscle cells of SHR. Kidney Int. 2002;61:2132–2141. doi: 10.1046/j.1523-1755.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- 342.Rogers T.B., Inesi G., Wade R., Lederer W. Use of thapsigargin to study Ca2+ homeostasis in cardiac cells. Biosci. Rep. 1995;15:341–349. doi: 10.1007/BF01788366. [DOI] [PubMed] [Google Scholar]