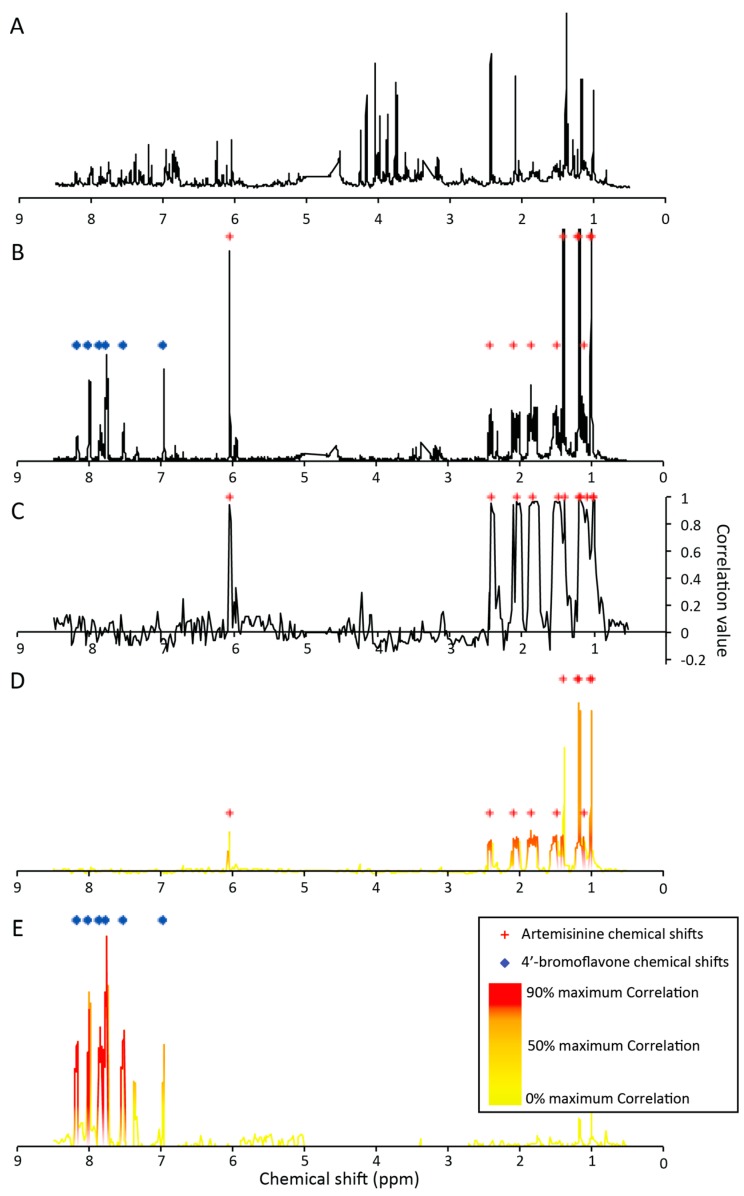
Figure 2.
Example of statistical deconvolution on the coelution of artemisinin and 4′-bromoflavone. (A) sum of all 80 1H-NMR spectra; (B) sum of all 80 1H-NMR spectra of fractions G9 to H10; (C) correlation 1H-NMR pseudospectrum, corresponding to feature RT = 2.18 min and m/z = 283.1566 (PI)—artemisinin as [M + H]+ adduct; (D) filtered 1H-NMR pseudospectrum corresponding to feature RT = 2.18 min and m/z = 283.1566 (PI)—artemisinin as [M + H]+ adduct; (E) filtered 1H-NMR pseudospectrum corresponding to feature RT = 2.37 min and m/z = 300.9882 (PI)—4′-bromoflavone as [M + H]+ adduct; red crosses () highlight chemical shifts detected for pure artemisinin; blue diamonds () highlight chemical shifts detected for pure 4′-bromoflavone.