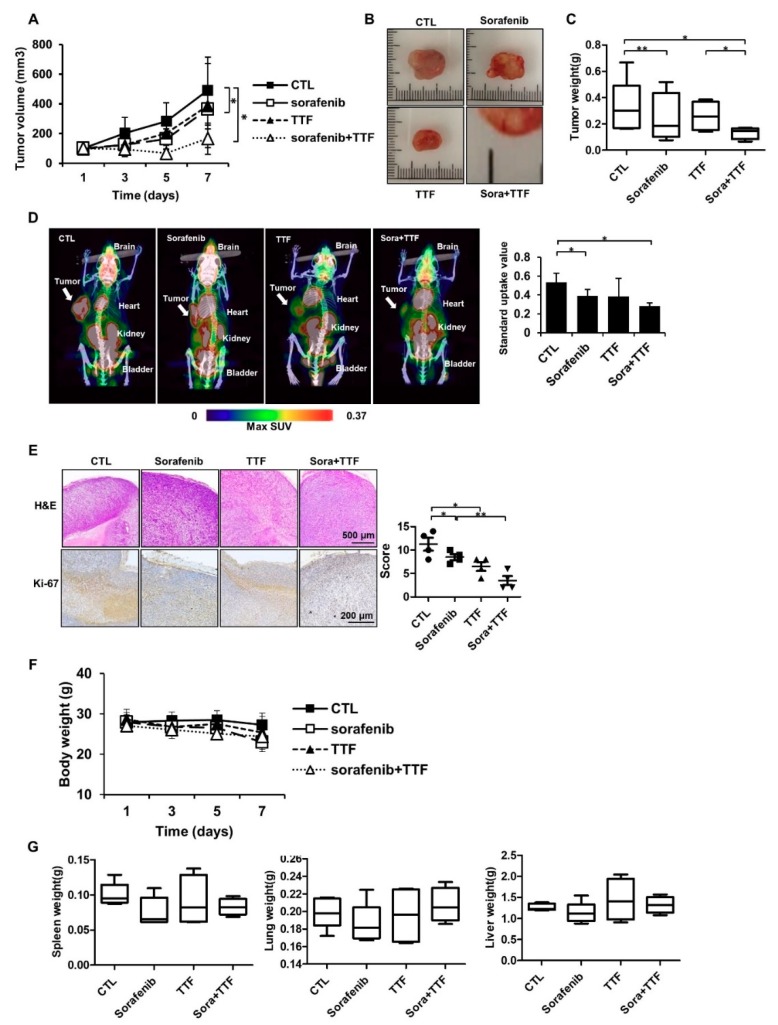
Figure 2.
Tumor-treating field (TTField)-sensitizing effects of sorafenib on glioblastoma in vivo. (A) Nude mice were inoculated with U373 cells and treated with TTFields, sorafenib, or a combination thereof. Tumor volumes were measured at the indicated time points, using the formula: volume = (length × width2 × 3.14)/6 (n = 8); * p < 0.05; (B) images of tumors isolated from control- or TTFields-treated mice, n = 4, Sora: sorafenib.; bar = 1 cm (C) tumors were excised and weighed at the end of the experiment (seven days). * p < 0.05; ** p < 0.01; (D) representative PET/CT images of U373 tumor-bearing mice after injection of [18F]-fluorodeoxyglucose (FDG). The radioactivity of [18F]-FDG in tumors is presented as the maximum standard uptake value (mean ± SD). * p < 0.05; SUV: Standard uptake value. (E) hematoxylin and eosin (H&E) staining and Ki-67 expression was examined by immunohistochemistry. * p < 0.05; ** p < 0.01, n = 4; Solid circle: Control; Solid square: Sorafenib; Triangle: Tumor treating fields; Inverted triangle: Sorafenib+TTF. (F) the body weights of the mice were not significantly different among the sorafenib-, TTFields-, and combination-treated groups, n = 4; (G) the spleen, liver, and lung tissues of the mice were excised and weighed at the end of the experiment (seven days), n = 4.