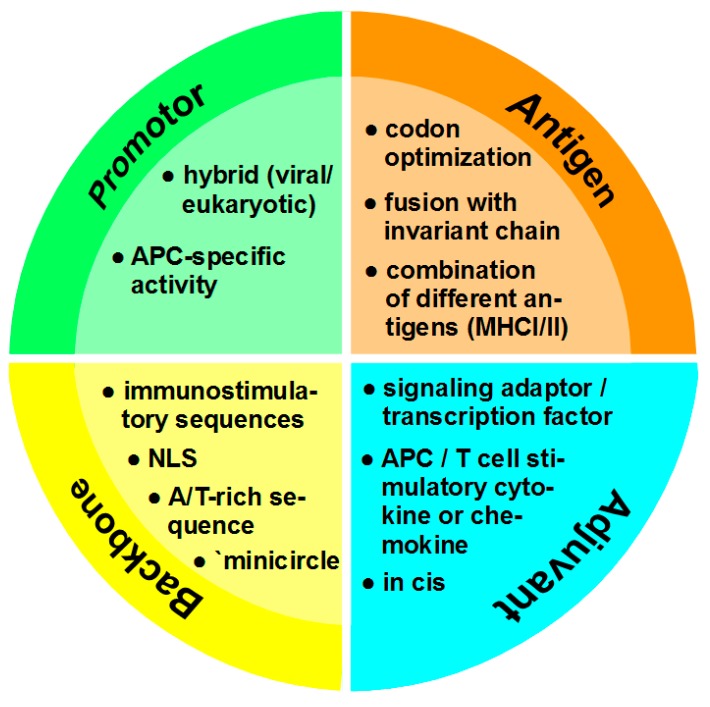
Figure 2.
Optimization parameters of DNA vaccine design. Use of a hybrid viral/eukaryotic promoter was shown to prevent transcriptional silencing as observed for viral promotors. Alternatively, a promoter engineered to transcriptinally target APC like DC may be used. To enhance antigen expression, especially in case of pathogens, codon optimization is important. To induce a broad CD4+/CD8+ T cell response, linker-separated sequences encoding different antigens may be used. In addition, fusion with a sequence encoding the invariant chain may enhance loading of antigen onto MHCII. Conventionally, molecular adjuvants intended to enhance the APC activation state and/or T cell attraction and polarization are encoded by expression vectors coadministered with a DNA vaccine. To ensure coexpression of antigen and a molecular adjuvant by a transfected cell, both sequences must be incorporated in the DNA vaccine (in cis), separated by an IRES or T2A sequence. The vector backbone comprises the part of the DNA vaccine which is not required for eukaryotic expression. Inherent or inserted immunostimulatory sequences are detected by danger receptors, and mediate APC activation. Inclusion of a NLS facilitates nuclear entry of the DNA. Intrinsic inhibitory effects of the backbone on the transfection efficiency are limited by insertion of A/T-rich sequences or by recombinase-mediated deletion of the prokaryotic part as a last step after propagation in bacteria to yield minicircle DNA.