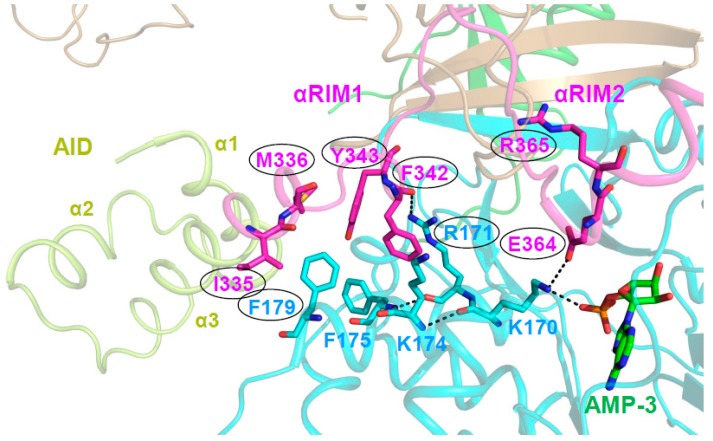
Figure 6.
αRIM2/CBS3 and AID-αRIM1/CBS2 interactions are linked. Structure of human AMP-bound AMPK α1β2γ1 (4RER) with key residues shown in a stick presentation; the α-linker is shown in magenta, the γ subunit in cyan, and the AID in light green. AMP bound at CBS3 and αRIM2 E364 directly interact with γ1 K170, which positions the αRIM1-binding residues R171, and indirectly through K174 and F175, F179, thus stabilizing the AID‒γ subunit interaction. Consistently, mutations of the αRIM1/γ subunit (and αRIM2/CBS3) interface residues highlighted by oval outlines (human α1: F342D/Y343D, I335D/M336D, E364, R365; γ1: R171A, F179D) are constitutively AMP-non-responsive. Dashed lines indicate hydrogen bonds.