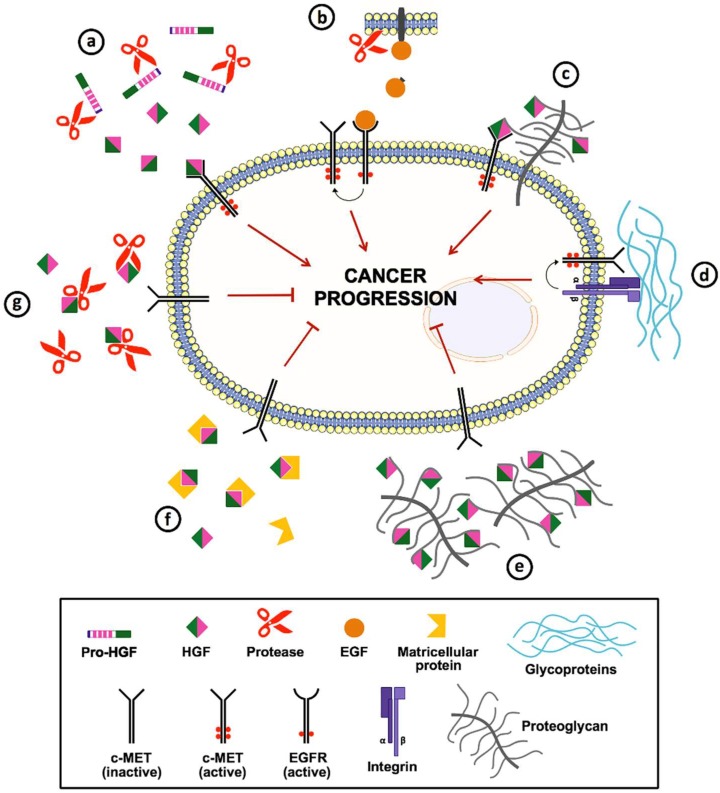
Figure 1.
Schematic representation of the extracellular matrix (ECM) components implicated in the regulation of c-MET signaling. (a) Extracellular proteases (e.g., HGFA, matriptase and MMP-2) are able to cleave and convert pro-hepatocyte growth factor (HGF) (single-chain inactive precursor) into its biologically active form. (b) Besides, some proteases (e.g., MMP-1, ADAM-17 and ADAMTS-1) mediate the shedding of EGFR ligands from the cell membrane; the activated EGFR can interact with c-MET (crosstalk), resulting in the latter’s ligand-free activation. (c) Cell surface heparan sulfate proteoglycans (e.g., CD44 and syndecan-1) bind to HGF and act as co-receptors and presenters for c-MET. (d) Glycoproteins (e.g., laminin and fibronectin) promote the association of integrins with c-MET, leading not only to ligand-free activation of c-MET but also to amplification of signaling by both receptors. (e) In contrast, some proteoglycans (e.g., heparin) can directly bind to HGF and prevent its interaction with c-MET. (f) Matricellular proteins (e.g., TSP-1) are another ECM components capable of sequestering HGF. (g) Also, specific proteases (e.g., ADAMTS-1) can sequester or degrade HGF, preventing the activation of HGF/c-MET signaling pathway.