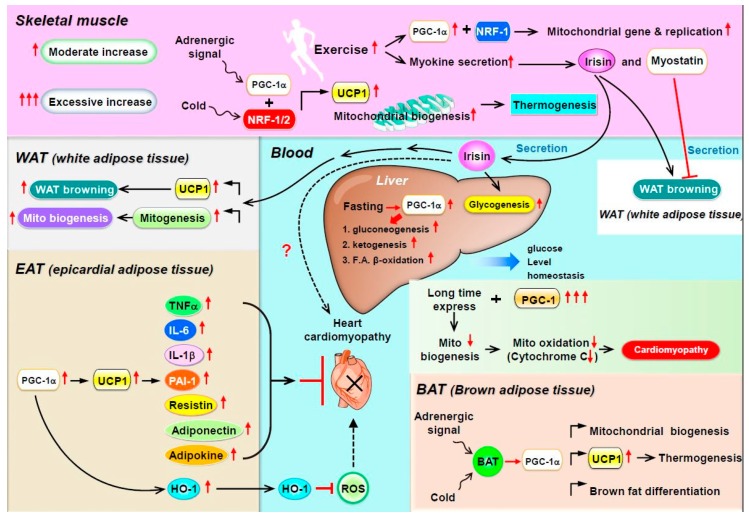
Figure 1.
Schematic description of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) function in various organs. Traditionally, in brown adipocytes (BAT) and white adipocytes (WAT), mitochondrial biogenesis and BAT gene expression are regulated by PGC-1α. Adrenergic stimulation and lower temperature trigger signaling cascades, including the upregulation of UCP-1 level, thereby resulting in body thermogenesis. In skeletal muscle and WAT, the transcriptional activity of PGC-1α is responsible for the expression of gene networks that control glucose uptake, glycolysis, fatty acid (FA) oxidation, tricarboxylic acid cycle, oxidative phosphorylation (OXPHOS), mitochondrial biogenesis, and protein uncoupling. Therefore, increasing exercise will increase mitochondrial gene biogenesis and secretion of myokines (such as irisin), which results in WAT browning and liver gluconeogenesis to prevent obesity and insulin resistance. In epicardial adipose tissue (EAT), increased heme oxygenase 1 (HO-1) expression depends on the PGC-1α–UCP-1 axis activity, which then decreases free radicals and reactive oxygen species (ROS) production, thus reducing cardiomyopathy. However, whether increased expression of cytokines such as TNF-α, IL-6, or adipokines by the PGC-1α–UCP-1 axis can reduce cardiomyopathy or not is still unclear.