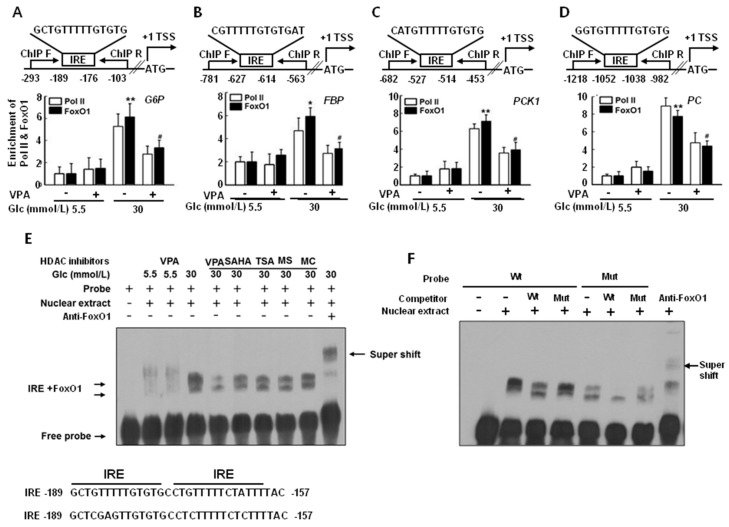
Figure 3.
Histone deacetylase (HDAC) inhibitors decrease recruitment of forkhead box O1 (FoxO1) to the cis-element of gluconeogenic genes. Recruitment of FoxO1 and polymerase II to glucose 6-phosphatase (G6P, (A)); fructose-1, 6-bisphosphatase (FBP, (B)); phosphoenolpyruvate carboxykinase (PCK1, (C)); and pyruvate carboxylase (PC, (D)) increased under hyperglycemic conditions (30 mmol/L glucose), an effect that was inhibited by administration of VPA (10 mmol/L). The graphs show the mean ± SEM of three independent experiments (* p < 0.05, ** p < 0.01 vs. vehicle in 5.5 mmol/L glucose; # p < 0.05 vs. vehicle 30 mmol/L glucose); (E) Electrophoretic mobility shift assay (EMSA) was performed using oligonucleotides of the insulin response element (IRE) as a probe. The probe formed complexes (arrow: →) with nuclear extract; these were decreased by VPA (10 mmol/L), suberoylanilide hydroxamic acid (SAHA, 10 μmol/L), trichostatin A (TSA, 1 μmol/L), MS275 (100 μmol/L), and MC1568 (10 μmol/L). HDAC inhibitors were treated for 24 h; (F) A 100-fold excess of the competitor, but not mutated probe, competed with the complex formation. Complexes that formed with the normal, but not mutated probes, were super-shifted by FoxO1 antibodies (arrow: ←).