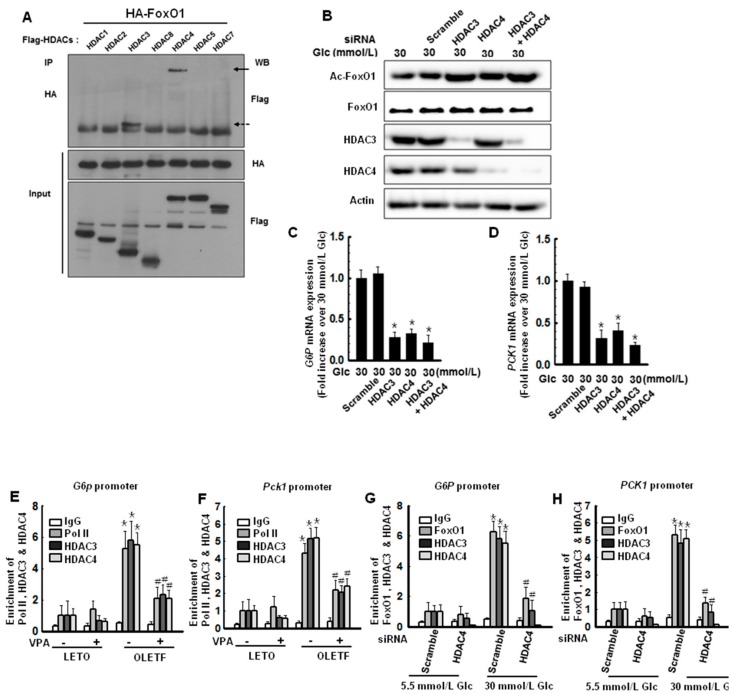
Figure 5.
Histone deacetylase 3 (HDAC3) and HDAC4 are responsible for FoxO1 deacetylation. (A) HepG2 cells were co-transfected with hemagglutinin (HA)-FoxO1 and either Flag-HDAC1, Flag-HDAC2, Flag-HDAC3, Flag-HDAC4, Flag-HDAC5, or Flag-HDAC7. FoxO1 was precipitated by anti-HA antibodies and FoxO1-interacting HDACs were detected by western blotting with an anti-Flag antibody; (B) HepG2 cells were transfected with HDAC3 siRNA, HDAC4 siRNA, or a combination for 48 h, resulting in increased FoxO1 acetylation. Knockdown of HDAC3, HDAC4, or a combination significantly attenuated the expression of G6P (C) and PCK1 (D); The graphs show the mean ± SEM of three independent experiments (* p < 0.05 vs. scrambled). Recruitment of Pol II, HDAC3 or HDAC4 on G6p promoter (E) or Pck1 promoter (F) was decreased when VPA was administered for 20 weeks in OLETF rats. The graphs show the mean ± SEM of six independent experiments (* p < 0.05 vs. LETO vehicle; # p < 0.05 vs. OLETF vehicle); Recruitment of FoxO1, HDAC3 or HDAC4 on G6P promoter (G) or PCK1 promoter (H) was decreased when HDAC4 was depleted by HDAC4 siRNA in HepG2 cells. The graphs show the mean ± SEM of three independent experiments (* p < 0.05 vs. 5.5 mM Glc scramble; # p < 0.05 vs. 30 mmol/L scramble). IP: immunoprecipitation.