Extended Data Fig. 3: Placement of β-bulges, β-turns and the tryptophan corner.
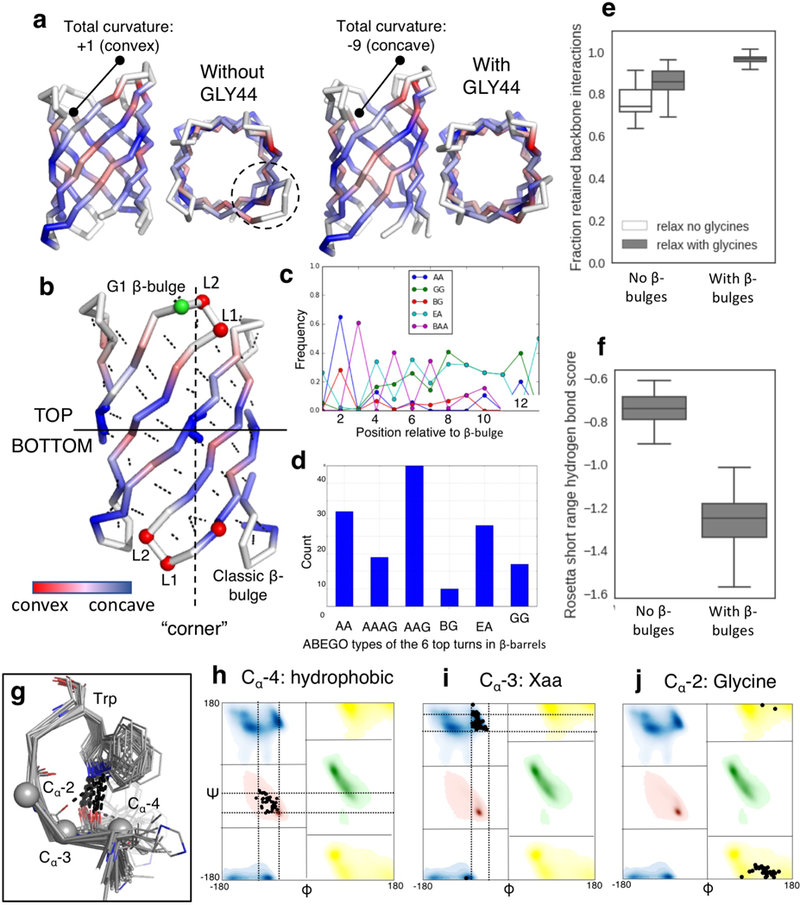
a, Change of curvature (from convex to concave) and protrusion (dashed circle) of the longest hairpin associated with the placement of a glycine kink at position 44. b, relationship between the “corners” in the β-sheet (dashed line) generated by the glycine kinks and the type and position of the β-bulges and β-turns (Supplementary methods). Cα are shown as spheres and colored by ABEGO type. The bottom of the barrel was defined as the side of the N- and C-termini. c, The type I β-turn (‘AA’ ABEGO type) is frequently found at the second position relative to a β-bulge in native proteins and was selected to connect bottom hairpins. d, This choice is further supported by the enrichment of type I (AA) turns over the canonical type I’ turn (GG) in native β-barrels (n=35 high resolution crystal structures). e&f, Poly-valine backbones built with β-bulges and the corresponding β-turns (n=194 independently generated models) retain more hydrogen bonds after relaxation than backbones built without β-bulges and with canonical type I’ β-turns (n=186 independently generated models) (e) and exhibit better scored hydrogen bonds per β-strand residue flanking the β-turns (f). Center line, median; box limits, upper and lower quartiles; whiskers, minimum and maximum values; points, outliers. g, Superposition of tryptophan corner motifs (n=41 high resolution crystal structures) extracted from native β-barrels. h-j, Amino acid preference and torsional constraints derived from the set and used to model the tryptophan corner. Bounded constraints limits are shown as dashed lines.
