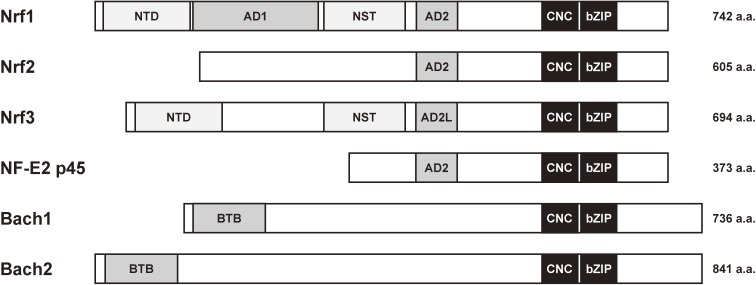
Figure 2.
Domain organization of CNC-bZIP family proteins. The image depicts the domain organization of six human CNC-bZIP proteins, namely, Nrf1, Nrf2, Nrf3, NF-E2 (p45 subunit), Bach1, and Bach2. All these proteins share cap ‘n’ collar (CNC) and a basic leucine zipper (bZIP) domains in their C-terminus region. Nrf1, Nrf2, Nrf3, and NF-E2 p45 contain one or two acidic domains (AD1, AD2, or AD2L) and regulate the transcriptional activation. In contrast, Bach1 and Bach2 are known as transcription repressors without acidic domains. However, they contain a broad-complex, tramtrack, bric-a-brac (BTB) domain. Nrf1 and Nrf3 exhibit a N-terminal domain (NTD) with a transmembrane region to anchor proteins to the ER membrane. Additionally, Nrf1 and Nrf3 contain an Asn/Ser/Thr-rich (NST) domain, which is a target for N-glycosylation.