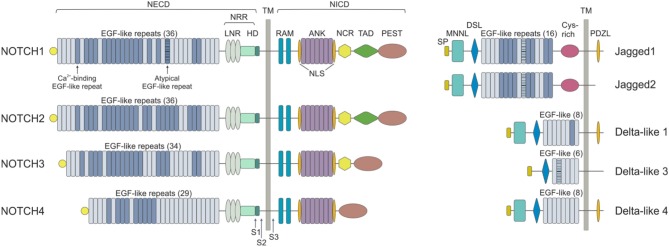
Figure 1.
NOTCH receptors and ligands. NOTCH receptors are structurally conserved type I proteins. There are four mammalian NOTCH receptors (NOTCH1-4) that contain multiple extracellular epidermal growth factor (EGF) repeats (from 29 to 36). Specific EGF repeats mediate ligand interactions. EGF repeats are followed by the negative regulatory region (NRR), which is composed of three cysteine-rich Lin repeats (LNR) and a heterodimerization domain (HD). NOTCH also contains a transmembrane domain (TM), an RBPJk associated module (RAM) domain, a nuclear localization sequences (NLS), a seven ankyrin repeats (ANK) domain, a NOTCH cytokine response (NCR) region, a transactivation domain (TAD) and a proline-glutamic acid-serine-threonin rich (PEST) domain. Mammalian NOTCH proteins are cleaved by furin-type convertases, which convert the NOTCH polypeptide into a NOTCH extracellular domain (NECD) and NOTCH intracellular domain (NICD) heterodimer that is connected by non-covalent interactions. After ligand binding, NOTCH is cleaved by metalloproteases and γ-secretase (S1, S2, and S3). NOTCH ligands can be divided into Jagged (Jagged 1 and Jagged 2) and Delta-like (DLL1, DLL3, and DLL4) groups on the basis of their domain composition. The extracellular domain of ligands is characterized by a NOTCH ligand N-terminal domain (MNNL), a Delta/Serrate/LAG-2 (DSL) domain, EGF motifs and a cysteine-rich (Cys) domain. DLLs lack this latter domain. The intracellular portion may contain a post-synaptic density protein ligand domain (PDZL).