Significance
Thymocyte-expressed molecule involved in selection (Themis) regulates T cell selection. Absence of Themis leads to severely reduced numbers of CD4 and CD8 T cells, indicating a defect in T cell selection. The molecular mechanism of Themis involvement is not clear. Themis was shown to bind to Src-homology domain containing phosphatase-1 (Shp1), which is a known negative regulator of T cell receptor signaling. Here, using a very sensitive technique to measure phosphatase activity from immunoprecipitated proteins, we find that Themis positively regulates Shp1 phosphatase activity in thymocytes. Shp1 activity is reduced in the absence of Themis, thus providing an explanation for why Themis-deficient thymocytes respond more strongly to positive-selecting ligands, resulting in fewer thymocytes reaching maturity.
Keywords: Themis, Shp1, T cell selection, phosphatase activity
Abstract
Thymocyte-expressed molecule involved in selection (Themis) has been shown to be important for T cell selection by setting the threshold for positive versus negative selection. Themis interacts with the protein tyrosine phosphatase (PTP) Src-homology domain containing phosphatase-1 (Shp1), a negative regulator of the T cell receptor (TCR) signaling cascade. However, how Themis regulates Shp1 is still not clear. Here, using a very sensitive phosphatase assay on ex vivo thymocytes, we have found that Themis enhances Shp1 phosphatase activity by increasing its phosphorylation. This positive regulation of Shp1 activity by Themis is found in thymocytes, but not in peripheral T cells. Shp1 activity is modulated by different affinity peptide MHC ligand binding in thymocytes. Themis is also associated with phosphatase activity, due to its constitutive interaction with Shp1. In the absence of Shp1 in thymocytes, Themis interacts with Shp2, which leads to almost normal thymic development in Shp1 conditional knockout (cKO) mice. Double deletion of both Themis and Shp1 leads to a thymic phenotype similar to that of Themis KO. These findings demonstrate unequivocally that Themis positively regulates Shp1 phosphatase activity in TCR-mediated signaling in developing thymocytes.
Thymocyte-expressed molecule involved in selection (Themis; also known as Themis1) was identified by several groups as a molecule important in the maturation of thymocytes, acting at the positive selection checkpoint (1–5, reviewed in refs. 6, 7). Themis is expressed in a stringently controlled manner during T cell development, and is most strongly expressed in the preselection CD4+CD8+ “double-positive” (DP) thymocytes before they are signaled for positive or negative selection. It is down-regulated after positive selection, and is expressed in mature thymocytes and T cells at a lower amount than in DP cells. Themis is tyrosine-phosphorylated within 30 s after T cell receptor (TCR) stimulation, suggesting that it may play a role early in signal transduction through the TCR (1, 8, 9). Themis interacts constitutively with Grb2, and after TCR stimulation, an interaction is induced with LAT (8). Themis has no classical conserved domains other than a proline-rich region and a potential bipartite nuclear localization sequence. A novel domain of unknown structure and function [cysteine-containing, all-β in Themis (CABIT)], has been identified in Themis, and Themis is predicted to fold into two CABIT domains (2, 10). Studies with Themis-deficient mice showed that Themis plays an important T cell-intrinsic role in regulating thymic selection (6, 7). The development of T cells is blocked at positive selection in Themis-deficient mice, where the gene is knocked out or mutated such that active protein is not produced. As a result, the number of CD4+ and CD8+ single-positive (SP) thymocytes is significantly reduced compared with wild-type (WT) mice. In the periphery, Themis-deficient mice have reduced numbers of both CD4+ and CD8+ T cells and a higher percentage of memory-phenotype T cells (CD62LloCD44hi), most likely due to lymphopenia-induced proliferation in the Themis knockout (KO) (1–7).
We previously reported that Themis controls the threshold between positive and negative selection of thymocytes (11). We found that Themis KO thymocytes did not discriminate correctly between weak and strong MHC-peptide (MHCp) ligands; hence, those MHCps that normally induce positive selection (antagonists and weak agonists) induce stronger signaling responses in Themis−/− thymocytes than in Themis+/+ cells (11). A similar result was found with stimulation by low amounts of anti-TCR antibodies (12). This effect has been thought to be mediated via interaction of Themis with Src-homology domain containing phosphatase-1 (Shp1) (11, 13).
Protein tyrosine kinases and protein tyrosine phosphatases (PTPs) are major cross-regulating elements in signal transduction pathways. Shp1 (encoded by the gene Ptpn6) is one such PTP that is expressed in all hematopoietic cells. Shp1 has two tandem Src homology-2 (SH2) domains near its amino (N) terminal. They act as protein phosphotyrosine-binding domains, allowing the interaction of this phosphatase with its substrates. Shp1 acts as an important negative regulator in both B and T cell signaling (14–17). Early studies using “motheaten” (Me/Me) mice that have a frameshift mutation near the 5′ end of the Shp1 coding sequence and “motheaten viable” (Mev/Mev) mice that have a different splicing mutation, resulting in very low phosphatase activity of Shp1, showed that these mice developed multiple disorders in the hematopoietic lineages. These multiple defects made it difficult to definitively identify the T cell-intrinsic effects of deletion of Shp1 (18–21). Loss of Shp1 activity has been linked to several autoimmune conditions and to susceptibility to experimental autoimmune encephalomyelitis in Me/Me mice (22). However, the importance of this phosphatase in T cell development is unclear, as conditional deletion of Shp1 in developing thymocytes led to undetectable (23) or rather subtle (24) defects in thymocyte development. The unimpaired thymocyte development in Shp1 conditional KO (cKO) mice is difficult to reconcile with the known role of Shp1 in regulation of TCR signaling pathways, suggesting functional redundancy between Shp1 and other phosphatases in the T cell lineage.
Themis interacts constitutively with Shp1 (11–13). They both interact constitutively with Grb2 (13, 25), and there is evidence both for (26) and against (13) the notion that Shp1 and Themis can interact with each other in the absence of Grb2. Shp1 binds to the N-terminal SH3 domain of Grb2, whereas Themis binds to its C-terminal SH3 domain (13, 25). The binding site on Shp1 remains undefined, but that on Themis is its polyproline region (25). We have previously proposed that the interaction of Themis with Shp1 is the mechanism for Themis being able to regulate TCR signaling in thymocytes (11). We previously hypothesized that Themis is a positive regulator of Shp1 phosphatase activity, but this model has recently been challenged by a publication indicating that Themis negatively regulates Shp1 activity (26).
To study how Themis and Shp1 regulate each other, we modified a tyrosine phosphatase assay available for purified proteins and optimized it to measure the PTP activity associated with ex vivo immunoprecipitated proteins. We find that Shp1 PTP activity is reduced in the absence of Themis, and that PTP activity coprecipitates with Themis. This effect is exclusive to thymocytes and not seen in peripheral T cells. This activity is lost after treatment with Shp1/Shp2-selective inhibitors.
Previous work using mice conditionally deficient for Shp1 in the T cell lineage, CD4-Cre, Ptpn6fl/fl (Shp1 cKO), reported a lack of any significant defect in thymocyte development (23). However, a more recent study has identified some relatively subtle changes in thymic selection (24, 27). We tested the Themis-associated PTP activity in Shp1 cKO thymocytes, finding that in the absence of Shp1, Themis interacts with Shp2 and retains most of the associated phosphatase activity. This demonstrates the possibility of redundancy between these different phosphatases for the interaction with Themis and explains the absence of severe defects in thymocyte development in Shp1 cKO mice. Furthermore, we bred Themis−/− mice to Shp1fl/fl CD4-Cre+ (Shp1 cKO) mice to have double deletion of both Themis and Shp1, and found that these mice have a very similar thymic phenotype to that of Themis−/− mice. Taken together, these data clearly show that Themis is a positive regulator of Shp1 activity in thymocytes.
Results
Use of Tyrosine Phosphatase Assay on Immunoprecipitated Proteins in ex Vivo Cells.
The basal tyrosine phosphorylation of Shp1 in thymocytes is reduced in Themis-deficient mice (11, 12). Shp1’s PTP enzymatic activity has been reported to be related to its tyrosine phosphorylation (28), although this has recently been called into question (26). To determine if Themis regulates the phosphatase activity of Shp1, we used an ex vivo tyrosine phosphatase assay to study the PTP activity associated with immunoprecipitated Shp1 and Themis in thymocytes. Since this assay has previously been used for purified proteins but not for immunoprecipitated proteins, it needed extensive optimization. We immunoprecipitated Shp1 and then added a universal phosphopeptide substrate [END(pY)INASL] that releases free phosphate after treatment with phosphatase. The amount of this free phosphate can be measured by absorbance using a plate reader. We first optimized the assay for immunoprecipitated Shp1 from 20 million thymocytes by trying different amounts of substrate. We chose 100 μM as the final concentration of peptide in the reaction (SI Appendix, Fig. S1A), and determined that 10 min is the appropriate incubation time for the phosphatase to act on the substrate and release free phosphate (SI Appendix, Fig. S1B). To check the sensitivity of the assay, we immunoprecipitated Shp1 from different numbers of cells and observed that this assay was sensitive enough to identify immunoprecipitated Shp1 from as few as 5 million thymocytes and that it was linear over a broad range of immunoprecipitated protein (5–35 × 106 cells) (SI Appendix, Fig. S1C).
Themis Regulates the Phosphatase Activity of Shp1 in Thymocytes.
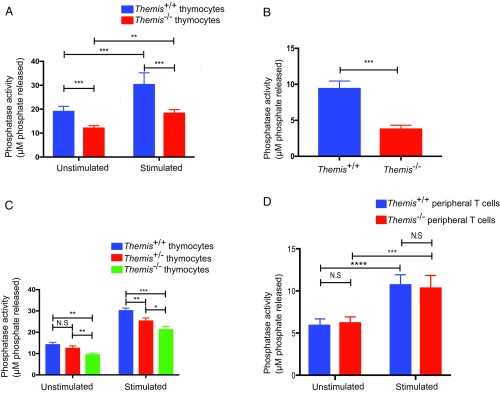
Given the interaction of Themis and Shp1, we measured the PTP activity associated with Shp1 in the presence and absence of Themis to see if Themis–Shp1 interaction is important for Shp1 activity. Themis+/+ and Themis−/− thymocytes were tested without stimulation or with a short (90 s) stimulation using cross-linking with streptavidin after coating the cells with biotinylated anti-CD3 and anti-CD4 antibodies. Shp1 was then immunoprecipitated, and a phosphatase assay was performed to measure the activity associated with Shp1. We found that Shp1 basal activity was significantly reduced in the absence of Themis (Fig. 1A). This effect was even more profound in the stimulated samples. This demonstrates that Shp1 PTP activity is positively regulated by Themis. Since thymus contains a mixed population of cells, which are both pre- and postselection, DP thymocytes that had low to intermediate expression of CD69 and TCR-β, which reflects the preselection population, were sorted and the Shp1 activity assay was performed. In Themis−/− preselection thymocytes, Shp1 activity was found to be significantly reduced compared with Themis+/+ preselection thymocytes, similar to what was observed with whole thymocytes (Fig. 1B). We also quantified Shp1 activity in thymocytes from Themis heterozygous mice and found that Shp1 activity was indeed regulated by the amount of Themis. Thymocytes from Themis+/− mice had lower activity compared with Themis+/+ thymocytes, but more than Themis−/− thymocytes (Fig. 1C). This unequivocally shows that Shp1 activity is positively regulated by Themis.
Fig. 1.
Phosphatase activity associated with Shp1 is reduced in Themis−/− thymocytes. (A) Tyrosine phosphatase activity assay of immunoprecipitated Shp1 on ex vivo thymocytes from Themis+/+ and Themis−/− mice with and without stimulation using biotinylated antibody cross-linking with streptavidin. (B) Tyrosine phosphatase activity assay of immunoprecipitated Shp1 on sorted ex vivo preselection thymocytes from Themis+/+ and Themis−/− mice with stimulation using biotinylated antibody cross-linking with streptavidin. (C) Titration of Themis by comparing Shp1 activity in unstimulated and stimulated thymocytes using biotinylated antibody cross-linking with streptavidin from Themis+/+, Themis+/−, and Themis−/− mice. (D) Tyrosine phosphatase activity assay of immunoprecipitated Shp1 on ex vivo sorted peripheral T cells from Themis+/+ and Themis−/− mice with and without stimulation using biotinylated antibody cross-linking with streptavidin. For sorting, two Themis+/+ and four Themis−/− mice were used. Data are representative of three experiments. Unpaired t test with mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. N.S., not significant.
Next, we examined if the effect of Themis on Shp1 phosphatase activity could also be observed in peripheral lymphocytes. As the Themis-deficient mice have a strongly reduced population of naive T cells, it was impractical to sort enough naive cells to immunoprecipitate (IP) Shp1 and perform the tyrosine phosphatase assay on them. Hence, we sorted all of the T cells, including both memory phenotype (CD44hi) and naive (CD44lo) T cells, to check the phosphatase activity associated with Shp1. We found that there was no significant difference in Shp1 phosphatase activity between Themis-sufficient and Themis-deficient peripheral T cells (Fig. 1D). These data suggest that Themis controls basal and TCR-dependent Shp1 activity in developing thymocytes, but that this is not the case (or at least to a much lesser extent) in mature T cells.
Since we observed that Shp1 PTP activity was increased in the presence of Themis in thymocytes but not in peripheral T cells, we next wanted to find how much of Shp1 is associated or not associated with Themis at the different stages of T cell development. We depleted Themis from thymocytes and from sorted peripheral T cells by sequential immunoprecipitation and measured the amount of the remaining free Shp1 using Western blotting. In thymocytes, we found that the majority of Shp1 was associated with Themis, leaving only about 25–30% free, and not associated with Themis (SI Appendix, Fig. S2A). In peripheral T cells, around 65–75% of Shp1 was free (SI Appendix, Fig. S2B). Similarly, we found that 45–55% of Shp1 was free in Themis heterozygous thymocytes (SI Appendix, Fig. S2C).
Themis Acts to Increase Phosphorylation of Shp1.
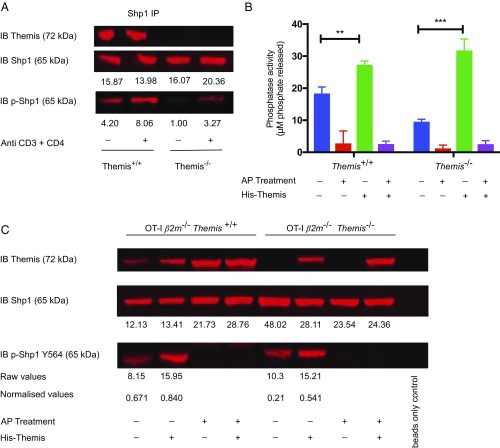
The reduced Shp1 phosphatase activity in the Themis KO correlated with reduced Shp1 phosphorylation (Fig. 2A), in agreement with previous studies (28–30) but in contrast to a recent publication (26). When we treated thymocytes with specific Shp1/2 inhibitors, we found consistently less phospho-Shp1 (p-Shp1) in both Themis WT and KO, whereas, in agreement with Choi et al. (26), treatment with pervanadate resulted in higher amounts of p-Shp1 (SI Appendix, Fig. S3). Shp1/2 inhibitors are known to deactivate Shp phosphatases by reducing their phosphorylation, which is known to be directly related to their activity (29). The reduced amount of p-Shp1 after specific inhibition of Shp1/Shp2, but its increase by pervanadate, which inactivates all of the phosphatases present in a cell, indicates that Shp1 is a substrate of phosphatases in general, but not simply of itself.
Fig. 2.
Themis acts to increase phosphorylation of Shp1. (A) Western blot with immunoprecipitated Shp1 assessing levels of p-Shp1 associated with Shp1 from Themis+/+ and Themis−/− thymocytes under both unstimulated and stimulated conditions using biotinylated antibody cross-linking with streptavidin. (B) Tyrosine phosphatase activity assay of immunoprecipitated Shp1 from OT-I β2m−/−Themis−/− and OT-I β2m−/−Themis+/+ thymocytes with or without additional recombinant His-tagged Themis in untreated and treated samples with AP for 1 h at 37 °C before doing the assay. **P < 0.005; ***P < 0.001. (C) Western blot with immunoprecipitated Shp1, assessing levels of p-Shp1 associated with Shp1 in similar samples as used for the phosphatase assay shown in B. IB, immunoblot. Raw values of p-Shp1 and Shp1 were calculated using LI-COR Odyssey software with the help of the background subtraction method. Normalized values were calculated by taking the ratio of raw values of p-Shp1 and Shp1 from the same samples.
Next, we wanted to study why loss of Themis leads to a decrease in phosphorylation of Shp1. Thymocytes from OT-I β2m−/− mice are blocked in development just before positive selection (similar to OT-I Tap−/−) due to the lack of cell surface expression of MHC class I molecules (31, 32). These developmentally blocked thymocytes are useful for analysis of thymocyte signal transduction (11, 32). We immunoprecipitated Shp1 from these thymocytes then treated with alkaline phosphatase (AP) for 1 h at 37 °C, and then quantified the activity of Shp1 in treated and untreated samples. We found that Shp1 lost its activity on treatment with AP, indicating that phosphorylation of Shp1 is crucial for its activity (Fig. 2B). In the same experiment, we also added purified recombinant His-tagged Themis in the thymocyte lysate from OT-I β2m−/−Themis−/− and OT-I β2m−/−Themis+/+ mice and, surprisingly, found that Shp1 activity was increased upon addition of Themis, but only in the sample that was not treated with AP (Fig. 2B). The sample that was treated with AP, however, did not show any change in activity even upon addition of Themis, suggesting that phosphorylation is crucial for Shp1 activity and that Themis regulates Shp1 phosphatase activity, through modulating its phosphorylation. Using Western blotting, we confirmed that addition of the exogenous Themis increased Shp1 tyrosine phosphorylation in AP-untreated lysates from both WT and Themis-deficient thymocytes (Fig. 2C). This increase in both Shp1 activity and tyrosine phosphorylation after addition of exogenous Themis further supports our hypothesis that Themis positively regulates Shp1 activity by increasing its tyrosine phosphorylation.
We investigated the localization of p-Shp1 and Shp1 in OT-I β2m−/−Themis+/+ and OT-I β2m−/−Themis−/− thymocytes stimulated with coverslips coated with CD3 antibody, using total internal reflection microscopy. The p-Shp1 was found to be lower in Themis KO thymocytes, but localization of p-Shp1 and Shp1 was similar in both genotypes, being close to the plasma membrane (SI Appendix, Fig. S4).
Themis-Associated Phosphatase Activity Is Due to Shp1 and/or Shp2.
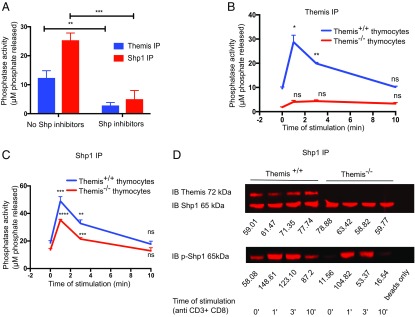
As Themis and Shp1 interact constitutively, we predicted that the immunoprecipitated Themis would associate with phosphatase activity due to coprecipitation of Shp1. We immunoprecipitated Shp1 and Themis from Themis+/+ thymocytes and looked for associated phosphatase activity (Fig. 3A). Themis-associated PTP activity was almost half of that of directly immunoprecipitated Shp1. To further confirm that the activity associated with Themis was due to its association with Shp1, we immunoprecipitated Themis and Shp1 from thymocyte lysates with 200 μM Shp1/Shp2 inhibitors for 30 min before doing the assay and found that no PTP activity was observed with immunoprecipitated Themis (Fig. 3A). We therefore concluded that Themis-associated phosphatase activity was indeed due to Themis interaction with Shp1 (and/or Shp2).
Fig. 3.
Phosphatase activity associated with Themis is due to its association with Shp1. (A) Tyrosine phosphatase activity assay with immunoprecipitated Themis and immunoprecipitated Shp1 from Themis+/+ thymocytes treated with or without Shp1/2 inhibitors (200 μM for 30 min before assay). (B) Tyrosine phosphatase activity assay assessing kinetics of phosphatase activity associated with immunoprecipitated Themis with different times of stimulation using biotinylated antibody cross-linking with streptavidin in Themis+/+ and Themis−/− thymocytes. All of the time points were compared with time 0 (i.e., unstimulated sample) for the purpose of statistical tests. (C) Tyrosine phosphatase activity assay with immunoprecipitated Shp1 with different times of stimulation using biotinylated antibody cross-linking with streptavidin in Themis+/+ and Themis−/− thymocytes. All of the time points were compared with time 0 (i.e., unstimulated sample) for the purpose of statistical tests. (D) Western blot showing levels of Themis (Top), Shp1 (Middle), and p-Shp1 (Bottom) associated with immunoprecipitated Shp1 from Themis+/+ and Themis−/− thymocytes with different times of stimulation. IB, immunoblot. Raw values of p-Shp1 and Shp1 were calculated using LI-COR Odyssey software with the help of the background subtraction method. Data are representative of three independent experiments. Unpaired t test with mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ns, not significant.
Next, we isolated thymocytes from Themis+/+ and Themis−/− mice and stimulated them using biotinylated anti-CD3ε and anti-CD4 antibodies, followed by cross-linking with streptavidin for different times, and then checked the IP for its phosphatase activity. We observed that the PTP activity associated with Themis increased rapidly and reached its peak at 1 min of stimulation. The phosphatase activity was drastically decreased by 3 min, and declined gradually to the level of the unstimulated sample by the end of 10 min (Fig. 3B). We performed similar experiments to study the kinetics of phosphorylation and phosphatase activity associated with Shp1, and found that, indeed, the kinetics were similar to those of Themis (Fig. 3 C and D).
Themis-Associated PTP Activity Correlates with Strength of TCR Signaling.
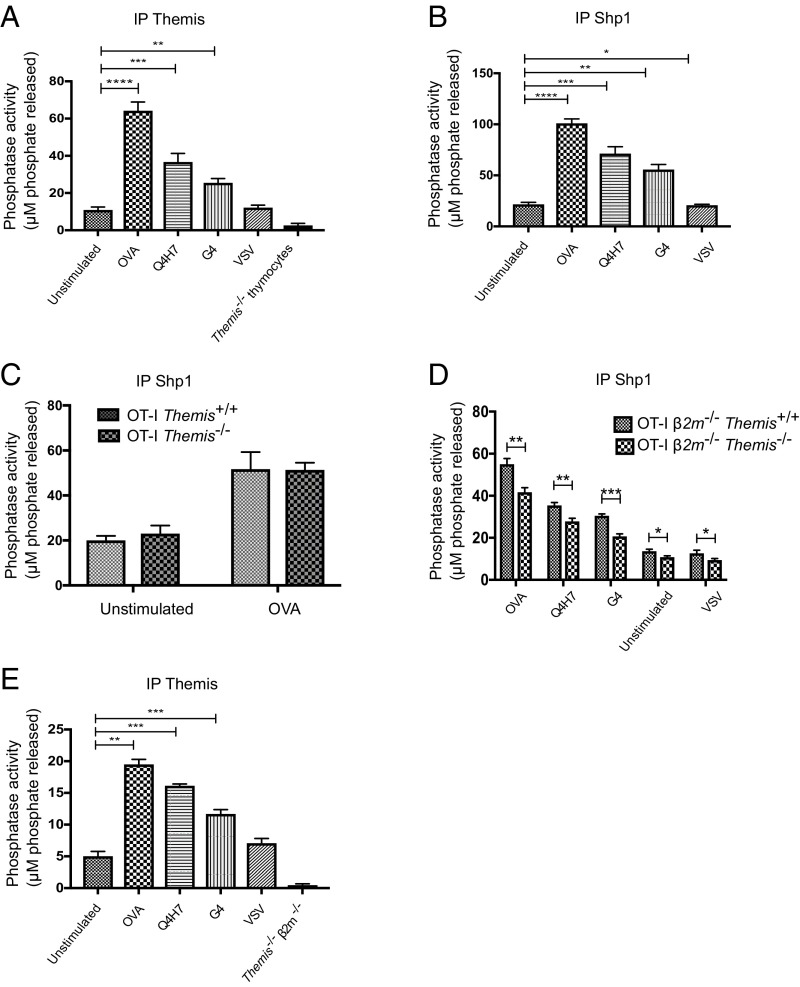
Next, we wanted to investigate Themis-associated PTP activity upon stimulation with different affinity peptide ligands. We used cytotoxic T lymphocytes (CTLs) cultured from splenocytes derived from TCR transgenic OT-I mice stimulated for 3 d with the antigenic ovalbumin (OVA) peptide. The antagonist and very weak agonist G4 and the somewhat stronger antagonist ligand Q4H7 both cause positive selection of OT-I thymocytes in fetal thymus organ culture, whereas the strong agonist OVA causes negative selection of thymocytes, and a vesicular stomatitis virus (VSV) peptide does not stimulate either thymocytes or mature OT-I T cells (31–33). We immunoprecipitated Shp1 and Themis from CTLs after stimulation with different peptide ligands. We observed that both the Themis and Shp1 immunoprecipitated samples had very similar correlations between phosphatase activity and TCR signal strength (Fig. 4 A and B). We next stimulated CTLs derived from splenocytes from OT-I Themis+/+ and OT-I Themis−/− mice cultured with OVA, observing that the phosphatase activity of the immunoprecipitated Shp1 was almost identical in the presence or absence of Themis (Fig. 4C). This further confirmed that Shp1 activity was unaltered by the absence of Themis in mature T cells.
Fig. 4.
Phosphatase activity associated with Themis and Shp1 in response to different affinity peptide ligands. Tyrosine phosphatase activity assay with (A) immunoprecipitated Themis from OT-I CTLs in response to different affinity peptide ligands using Themis−/− thymocytes as a negative control. (B) Immunoprecipitated Shp1 from OT-I CTLs in response to the use of different affinity peptide ligands along with unstimulated CTLs. (C) Immunoprecipitated Shp1 from CTLs made from OT-I Themis+/+ and OT-I Themis−/−, comparing unstimulated and OVA-stimulated cells. (D) Immunoprecipitated Shp1, showing reduction in Shp1 activity in OT-I β2m−/−Themis−/− thymocytes compared with OT-I β2m−/−Themis+/+ thymocytes in response to different stimulations along with unstimulated control. (E) Immunoprecipitated Themis in OT-I β2m−/−Themis+/+ thymocytes in response to stimulations using different affinity peptide ligands and OT-I β2m−/−Themis−/− thymocytes as a negative control. All of the stimulations were done for 90 s. Data are representative of three independent experiments. Unpaired t test with mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We were then interested to see what happens to Shp1 activity in thymocytes in response to different affinity peptide ligands. We stimulated thymocytes from OT-I β2m−/−Themis+/+ and OT-I β2m−/−Themis−/− mice using Kb-tetramers with various peptides, as above, and measured the phosphatase activity associated with Shp1 and Themis (Fig. 4 D and E). We found that Shp1 activity was significantly reduced in all of the samples in the absence of Themis (Fig. 4D), which is similar to what we saw in B6 Themis−/− thymocytes (Fig. 1A). Phosphatase activity associated with Themis was different for each stimulation, and OVA stimulation gave the highest PTP activity, with significantly less activity for the weaker ligands Q4H7 and G4. However, the amount of Themis that was associated with Shp1 was similar in all of the stimulated samples (SI Appendix, Fig. S5). Thus, it was the activity of Shp1 that was different in response to different affinity ligands, and Themis regulated this activity.
Deletion of Shp1 in Themis−/− Mice Has No Effect on Thymocyte Development.
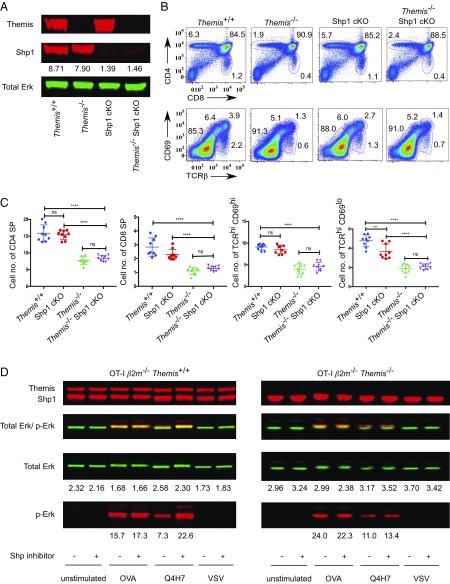
Since we found that Themis is a positive regulator of Shp1, it was expected that deletion of Shp1 in Themis−/− mice would lead to similar a thymic defect as seen in Themis−/− thymocytes. However, this is in contrast to a recent study (26), which showed that deletion of both Themis and Shp1 in the same mice leads to rescue of the thymic defect that is seen in Themis−/− mice. To address this contradiction, we bred Shp1fl/fl CD4-Cre+ (Shp1 cKO) mice with Themis−/− mice to get double deletion of both Themis and Shp1 in the same mice. We analyzed thymocytes of Themis−/− Shp1 cKO mice along with Themis−/−, Themis+/+, and Shp1 cKO mice (Fig. 5A). We found that thymic development of Themis−/− Shp1 cKO mice was very similar to that of Themis−/− mice and was significantly different from Themis+/+ and Shp1 cKO mice (Fig. 5B). We further analyzed thymocytes in Themis−/− Shp1 cKO mice based on the expression of TCR-β and CD69, and found that all of the subpopulations were similar to that of Themis−/− thymocytes (Fig. 5 B and C). This clearly indicated that Themis is not a negative regulator of Shp1 activity in thymocytes, as has been suggested. To further validate the role of Themis in the regulation of Shp1 activity, we treated OT-I β2m−/−Themis+/+ and OT-I β2m−/−Themis−/− thymocytes with 200 μM Shp inhibitors for 1 h at 37 °C and then stimulated them with Kb-OVA, Q4H7, and VSV tetramers. We found that the OT-I β2m−/−Themis+/+ thymocytes that were treated with the inhibitors had a significant increase in p-Erk on stimulation with Q4H7, whereas there was no increase in OT-I β2m−/−Themis−/− thymocytes (Fig. 5D). This indicates that Themis-deficient thymocytes have lower levels of Shp1 activity, making them relatively resistant to Shp1 inhibition (11).
Fig. 5.
Thymic phenotype of Themis−/− Shp1 cKO mice is similar to that of Themis−/− mice. (A) Western blot showing amounts of Themis and Shp1 in thymocytes of Themis+/+, Themis−/−, Shp1fl/fl CD4-Cre+ (Shp1 cKO), and Themis−/− Shp1 cKO mice. Total Erk is used as a loading control. Shp1 was quantified by normalizing to total Erk. (B) Representative flow cytometry showing different subpopulations of thymus (Top) and thymus maturation based on CD69 and TCR-β (Bottom) in Themis+/+, Shp1 cKO, Themis−/−, and Themis−/− Shp1 cKO thymocytes. (C) Graphs showing enumeration of total number (*106) of CD4 SP, CD8 SP, TCRhiCD69hi, and TCRhiCD69lo populations in the thymus in Themis+/+, Shp1 cKO, Themis−/−, and Themis−/− Shp1 cKO mice. Data are representative of five experiments. There were three to four mice per genotype of the same age and sex in each experiment. Unpaired t test with mean ± SD. **P < 0.01; ****P < 0.0001. ns, not significant. (D) Western blot showing levels of p-Erk on stimulation with Kb tetramers in Shp inhibitor-treated and untreated thymocytes from OT-I β2m−/−Themis+/+ and OT-I β2m−/−Themis−/− mice. Raw values of proteins were calculated using LI-COR Odyssey software with the help of the background subtraction method.
We then looked at the periphery of these mice. Interestingly, we found that there was partial rescue of peripheral CD4 and CD8 T cells, which looked intermediate to that of Themis−/− and Themis+/+ peripheral T cells (SI Appendix, Fig. S6 A and B). Also, peripheral CD8 T cells had more memory phenotype cells (SI Appendix, Fig. S6 C and D), similar to Shp1 cKO mice, which could be due to IL-4 homeostasis in the absence of Shp1 (23). Expression of CD44 and CD62L in CD4 T cells in Themis−/− Shp1 cKO mice also looked similar to that in Shp1 cKO mice. These findings strongly suggest that Themis has different functions in the thymus versus periphery.
Themis–Shp2 Interaction Corrects for Shp1 Deficiency in Thymocyte Development.
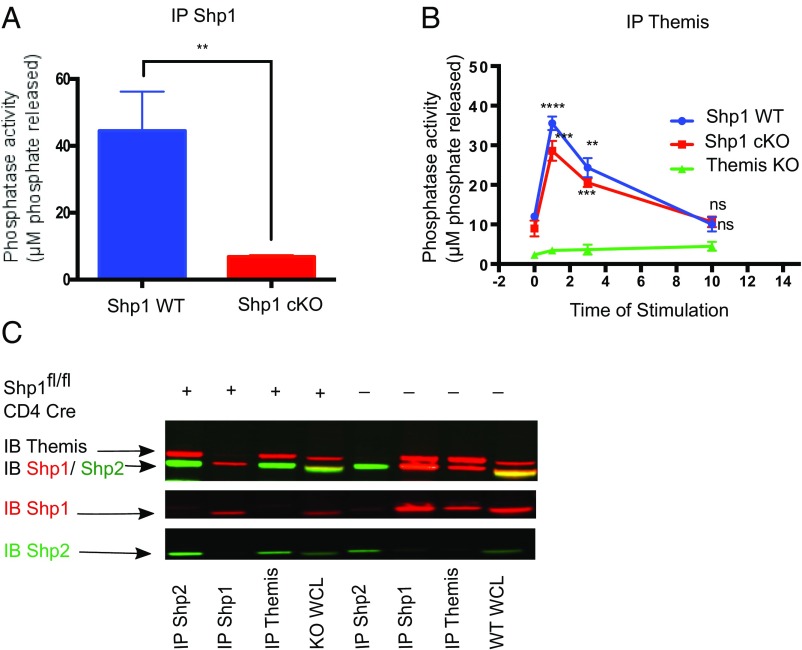
The role of Shp1 in the development of thymocytes is not yet clear. There have been contradictory studies regarding the involvement of this phosphatase in thymic development (23, 24, 27). As Themis regulates Shp1 activity in the thymus, we initially predicted the phenotype of Shp1-deficient thymocytes to be similar to that of Themis KO mice. However, we observe only a subtle thymocyte developmental defect in Shp1 cKO mice (Fig. 5B), consistent with previous reports (24). We previously suggested that Themis can interact with the related Shp2 phosphatase or other phosphatases, and we have indeed found some evidence for this in overexpression studies using cell lines (11) and by mass spectroscopy in Jurkat 1G4-CD8+ cells (13). Other researchers, using mass spectroscopy, found that Themis associated with Shp2; however, the association value was extremely weak (12). We therefore tested for interactions between Themis and Shp2 in WT and Shp1 cKO thymocytes by coimmunoprecipitation and Western blotting. First, we confirmed that there was no activity associated with Shp1 in Shp1 cKO thymocytes (Fig. 6A). Then, to check whether Themis is associated with phosphatase activity in the absence of Shp1, we immunoprecipitated Themis from Shp1 WT and Shp1 cKO thymocytes and tested for coprecipitated phosphatase activity. We found that Themis was indeed associated with a significant amount of phosphatase activity in Shp1 cKO thymocytes. We performed a similar kinetic experiment as described in Fig. 3B above, observing that Themis-associated phosphatase activity in Shp1 cKO mice follows a similar pattern as it does in Shp1 WT mice (Fig. 6B).
Fig. 6.
Themis interacts with Shp2 when Shp1 is genetically deleted. (A) Tyrosine phosphatase assay with immunoprecipitated Shp1 in Shp1 WT and Shp1 cKO thymocytes. (B) Tyrosine phosphatase assay with immunoprecipitated Themis with different times of stimulation using biotinylated antibody cross-linking with streptavidin in WT and Shp1 cKO thymocytes, using Themis−/− thymocytes as a negative control. All of the time points were compared with unstimulated (i.e., time 0) for the purpose of statistics. (C) Western blot showing (Upper), Themis (∼72 kDa red band) interaction with Shp2 (∼65 kDa green band) in Shp1 cKO thymocytes (first and third columns from left) and with Shp1 (∼65 kDa red band) in Shp1 WT thymocytes (sixth and seventh columns from left). The Middle and Lower show Shp1 (red) and Shp2 (green) separately for better visualization. IB, immunoblot. Unpaired t test with mean ± SD. **P < 0.01; ***P < 0.001; ****P < 0.0001. ns, not significant.
In the Shp1 cKO thymocytes, immunoprecipitation with anti-Themis coprecipitated Shp2, whereas this interaction was undetectable in WT thymocytes (Fig. 6C). Conversely, only Shp1 was detectable from the Themis IP from WT cells. Anti-Shp2 coprecipitated Themis from Shp1 cKO thymocytes but not from WT, and anti-Shp1 coprecipitated Themis from WT. These results show redundancy for Themis binding between Shp1 and Shp2 and suggest that Shp2 can compensate for Shp1 deficiency, resulting in relatively normal thymocyte development in the Shp1 cKO mice.
Discussion
Themis has been shown to have an important role in the development of T cells (1–5). However, the underlying mechanism remains unclear. Using TCR transgenic preselection thymocytes, we previously showed that in the absence of Themis, cells are not able to distinguish between weak positively selecting ligands and strong negatively selecting ligands in terms of their responding signal strength (11). The data presented here showing reduction of Shp1 phosphatase activity in Themis-deficient thymocytes provide a molecular mechanism for the observed increase in TCR signaling in response to weak ligands. Reduced Shp1 phosphatase activity is predicted to lead to stronger TCR signals in the absence of Themis. Ca2+ and p-Erk responses, as well as more TCR-proximal responses such as LAT phosphorylation and PLC-γ1 activation, are stronger in the KO thymocytes compared with WT in response to weak TCR ligands (11) or to suboptimal anti-TCR antibody stimulation (12). This is particularly evident with weak, positive-selection ligands, leading to increased activation of markers of apoptosis (11, 13).
To find out the role of Themis in regulation of Shp1 phosphatase activity, we used a very sensitive ex vivo tyrosine phosphatase assay to measure the PTP activity associated with immunoprecipitated proteins either directly (like Shp1 itself) or indirectly, for example, by immunoprecipitating Themis and measuring coimmunoprecipitated PTP activity. We found that Themis is associated with Shp1- and/or Shp2-dependent phosphatase activity. Critically, Shp1 phosphatase activity is severely reduced in Themis-deficient thymocytes, consistent with a role for Themis in positive regulation of Shp1 activity. We also showed that Themis controls Shp1 activity in thymocytes, particularly in relation to TCR stimulation. We were able to detect PTP activity reproducibly in either anti-Shp1 or anti-Themis IP, and a Shp1/2-specific inhibitor blocked this activity. Our data show an increase in Shp1 phosphatase activity after TCR stimulation, and this is severely reduced in Themis-deficient thymocytes. We further demonstrated that Themis increases phosphorylation of Shp1. On treating thymocytes with AP, Shp1 is no longer able to be phosphorylated and loses its activity. Interestingly, addition of purified His-tagged Themis aids the phosphatase activity of Shp1 and also increases phosphorylation of Shp1 in the untreated thymocytes. These findings clearly indicate that Themis increases the phosphatase activity of Shp1 by increasing its phosphorylation. We propose a model for how Themis binding leads to activation of Shp1 (SI Appendix, Fig. S7). Themis has been reported to bind to the phosphatase domain of Shp1 (26). We propose that Themis might act to reduce the inhibition by binding of the N-SH2 domain to the phosphatase domain, or by opening the active site, thus resulting in higher phosphatase activity. In the absence of Themis, Shp1 is inactive, with its N-SH2 domain bound to the phosphatase domain (34) (S7A), and, as shown in this paper, when Themis is bound to Shp1, it has the effect of increasing Shp1’s phosphatase activity (S7B) and increasing its phosphorylation (S7C). The phosphorylation of Y536 and Y564 leads to an increase in phosphatase activity, with Y536 binding to the N-SH2 domain (28).
The phosphatase activity of Shp1, and that associated with Themis, is affected by the TCR signal strength. Stimulation of OT-I β2m−/−Themis+/+ preselection thymocytes with peptide ligands of different affinities resulted in graduated amounts of phosphatase activity immunoprecipitated with Themis or Shp1. As the affinity of MHCp ligand for the TCR decreased, the phosphatase activity associated with Themis or Shp1 also decreased. In our system, in OT-I β2m−/−Themis−/− thymocytes, Shp1 PTP activity was significantly reduced compared with the OT-I β2m−/−Themis+/+ cells in response to all of the different affinity peptide ligands. This strongly supports the hypothesis that the signaling defects in Themis−/− thymocytes (11) are due to reduced activity of Shp1.
Shp1 has similar PTP activity in peripheral T cells from Themis+/+ and Themis−/− mice, which indicates that Shp1 activity in the periphery is not affected by the absence of Themis. The patterns of phosphatase activity associated with Themis and Shp1 in response to different affinity peptide ligands were very similar, indicating that Themis-associated phosphatase activity is due to its interaction with Shp1. It is very interesting that a general effect on Shp1 activity is seen only in thymocytes and not in peripheral T cells, suggesting that Themis plays different roles in the thymus and the periphery. One factor that should be considered here is the relative amounts of these proteins in the peripheral T cells compared with thymocytes, since Themis is most strongly expressed in immature preselection DP thymocytes and much less expressed in peripheral T cells. This could enable Themis to control Shp1 activation and/or its ability to access the TCR signaling machinery only at that specific time in T cell development. Interestingly, using sequential immunoprecipitation, we found that much more Shp1 is associated with Themis in thymocytes compared with peripheral T cells. However, in CTLs, Themis is expressed in a much higher amount compared with that of naive peripheral lymphocytes, and a significant amount of Shp1 is bound to Themis, leading to phosphatase activity associated with Themis. Irrespective of that, Shp1 activity is similar in Themis-sufficient or -deficient CTLs. This clearly indicates that Themis does play different roles in thymocytes versus peripheral T cells.
Currently, there are two models of how Themis functions in T cell selection. We have previously proposed that Themis acts as a negative regulator of TCR signal strength during thymic selection, attenuating signals from low- and intermediate-affinity MHCp ligands to allow the developing thymocytes to pass through the positive selection checkpoint (11). Since Themis interacts with Shp1, and Themis-deficient thymocytes show reduced Shp1 phosphorylation, we suggested that Themis is a positive regulator of Shp1 phosphatase activity. The data presented in this paper strongly support this hypothesis.
An alternative model for the action of Themis during thymic selection has recently been proposed by Choi et al. (26). According to this model, Themis is a negative regulator of Shp1 phosphatase activity through reactive oxygen species (ROS)-dependent regulation of Shp1. Both models agree on the critical role of Themis in regulation of Shp1 phosphatase activity, but they propose strikingly different predictions about Shp1 phosphatase activity in Themis-deficient cells. Choi et al. (26) proposed that Themis is involved in regulating the oxidation state of Shp1’s active site in the presence of ROS, and thus inactivating Shp1. However, it has been shown that thymocyte stimulation does not lead to production of ROS (35), questioning the relevance of this proposed mechanism. Choi et al. (26) also performed a Shp1 activity assay similar to that reported here, but using purified proteins. It is not clear where the ROS would come from in such a cell-free system. The experimental system used by Choi et al. (26) does not recapitulate physiologically relevant aspects of regulation of Shp1 activity, such as changes in phosphorylation and particularly subcellular localization and protein–protein interactions. Our data were obtained using ex vivo cells, which is a more biologically relevant system.
To support the data of Themis being a negative regulator of Shp1, Choi et al. (26) made Themis and Shp1 double-KO mice and found a complete rescue of the thymic defect seen in Themis−/− thymocytes and peripheral T cells. This is in direct contrast to our findings. To address these issues, we bred similar mice and analyzed them along with Themis+/+, Themis−/−, and Shp1fl/fl CD4-Cre+ (Shp1 cKO) mice of the same age and sex. Previous work using mice conditionally deficient in the T cell lineage for Shp1 (Shp1fl/fl CD4-Cre+) reported a lack of any significant defect in thymocyte development (23). However, a more recent study has identified some relatively subtle changes in thymic selection (24) in mice of the same genotype by performing a more in-depth analysis of thymocyte development.
Thymocytes develop through well-defined stages. During positive selection, DP thymocytes expressing both CD4 and CD8 markers up-regulate CD69. Those DP thymocytes that get selected also up-regulate TCR-β to intermediate and high levels after selection and down-regulate CD8 coreceptor expression. These are the cells that further mature into CD4 or CD8 SP subsets. Initially these cells have high coexpression of CD69 and TCR (TCRhiCD69hi population), but they down-regulate CD69 (TCRhiCD69lo population) during maturation to allow migration from the thymus (6, 36). Martinez et al. (24) did a detailed analysis and observed that the TCRhiCD69lo population from Shp1-deficient mice had reduced numbers of CD4+CD8int, CD8 SP, and CD4 SP cells. Consistent with their results, we found subtle differences in the TCRhiCD69lo population in Shp1 cKO mice compared with WT mice. One possible explanation of the relatively unperturbed thymic development in Shp1 cKO was redundancy of Shp1 with other phosphatases, such as Shp2. We showed that the Themis–Shp2 interaction replaces the Themis–Shp1 interaction in the absence of Shp1. This can explain the less severe phenotype of Shp1 cKO.
Furthermore, we consistently found that Themis−/− Shp1 cKO mice had a very similar thymic phenotype to that of Themis−/− mice. We did not observe any rescue of the thymic defect in these mice. This contrasts to what Choi et al. (26) reported, possibly due to the larger number of replicates in our study. Consistently, Themis−/− Shp1 cKO mice, which had deletion of both Themis and Shp1 in the same mice, were found to have no defect in the thymus, clearly indicating that Themis acts as positive regulator of Shp1. These results were further supported by a significant increase in p-Erk after stimulation with weak ligands like Q4H7 in Shp inhibitor-treated OT-I β2m−/−Themis+/+ thymocytes, but not in OT-I β2m−/−Themis−/− thymocytes. This is because in OT-I β2m−/−Themis−/− thymocytes, Shp1 activity is already low (due to lack of Themis) and further treatment with Shp inhibitors does not make much of a difference. On the other hand, OT-I β2m−/−Themis+/+ thymocytes have higher activity of Shp1; upon treatment with Shp inhibitors, Shp1 activity is drastically reduced, leading to stronger signaling events.
However, in peripheral T cells, double deletion of both Themis and Shp1 leads to partial rescue of the phenotype. Total numbers of CD4 and CD8 T cells in the periphery of the doubly deficient mice were significantly higher than in Themis−/− mice, although lower than in Themis+/+ mice. CD8 T cells have a similar memory phenotype to that of Shp1 cKO mice, as shown previously (23). These findings strongly suggest that the role of Themis is different in the thymus versus the periphery. Nevertheless, it may also be possible that Themis is involved in both negative and positive regulation of TCR signaling under different conditions. Further studies will be required to completely understand the differences in the role of Themis in T cells.
Materials and Methods
Details of all materials and methods, including information on mice, immunoprecipitation and Western blotting, phosphatase assay, antibodies used, sequential immunoprecipitation, AP treatment, recombinant Themis, total internal reflection microscopy, flow cytometry, cell sorting, and statistical analysis, are provided in SI Appendix. All animal work was approved by Animal Care and Use Committee of National University of Singapore.
Supplementary Material
Acknowledgments
This work was supported by the Singapore Ministry of Health’s National Medical Research Council (NMRC) under NMRC CBRG15may017 and by Singapore Ministry of Education Grant 2014-T2-1-136 (to N.R.J.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720209115/-/DCSupplemental.
References
- 1.Fu G, et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol. 2009;10:848–856. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesourne R, et al. Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol. 2009;10:840–847. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson AL, et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat Immunol. 2009;10:831–839. doi: 10.1038/ni.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakugawa K, et al. A novel gene essential for the development of single positive thymocytes. Mol Cell Biol. 2009;29:5128–5135. doi: 10.1128/MCB.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick MS, et al. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proc Natl Acad Sci USA. 2009;106:16345–16350. doi: 10.1073/pnas.0908593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gascoigne NRJ, Rybakin V, Acuto O, Brzostek J. TCR signal strength and T cell development. Annu Rev Cell Dev Biol. 2016;32:327–348. doi: 10.1146/annurev-cellbio-111315-125324. [DOI] [PubMed] [Google Scholar]
- 7.Gascoigne NRJ, Acuto O. THEMIS: A critical TCR signal regulator for ligand discrimination. Curr Opin Immunol. 2015;33:86–92. doi: 10.1016/j.coi.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Brockmeyer C, et al. T cell receptor (TCR)-induced tyrosine phosphorylation dynamics identifies THEMIS as a new TCR signalosome component. J Biol Chem. 2011;286:7535–7547. doi: 10.1074/jbc.M110.201236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesourne R, et al. Interchangeability of Themis1 and Themis2 in thymocyte development reveals two related proteins with conserved molecular function. J Immunol. 2012;189:1154–1161. doi: 10.4049/jimmunol.1200123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada T, et al. Differential function of Themis CABIT domains during T cell development. PLoS One. 2014;9:e89115. doi: 10.1371/journal.pone.0089115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu G, et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature. 2013;504:441–445. doi: 10.1038/nature12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zvezdova E, et al. Themis1 enhances T cell receptor signaling during thymocyte development by promoting Vav1 activity and Grb2 stability. Sci Signal. 2016;9:ra51. doi: 10.1126/scisignal.aad1576. [DOI] [PubMed] [Google Scholar]
- 13.Paster W, et al. A THEMIS:SHP1 complex promotes T-cell survival. EMBO J. 2015;34:393–409. doi: 10.15252/embj.201387725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pani G, Siminovitch KA. Protein tyrosine phosphatase roles in the regulation of lymphocyte signaling. Clin Immunol Immunopathol. 1997;84:1–16. doi: 10.1006/clin.1996.4326. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz U, Ravichandran KS, Burakoff SJ, Neel BG. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc Natl Acad Sci USA. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J Exp Med. 1995;181:2077–2084. doi: 10.1084/jem.181.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siminovitch KA, Neel BG. Regulation of B cell signal transduction by SH2-containing protein-tyrosine phosphatases. Semin Immunol. 1998;10:329–347. doi: 10.1006/smim.1998.0125. [DOI] [PubMed] [Google Scholar]
- 18.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 19.Shultz LD, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 20.Shultz LD, Sidman CL. Genetically determined murine models of immunodeficiency. Annu Rev Immunol. 1987;5:367–403. doi: 10.1146/annurev.iy.05.040187.002055. [DOI] [PubMed] [Google Scholar]
- 21.Kozlowski M, et al. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med. 1993;178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng C, et al. Expression of the tyrosine phosphatase SRC homology 2 domain-containing protein tyrosine phosphatase 1 determines T cell activation threshold and severity of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:4511–4518. doi: 10.4049/jimmunol.168.9.4511. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DJ, et al. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J Exp Med. 2013;210:1419–1431. doi: 10.1084/jem.20122239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez RJ, Morris AB, Neeld DK, Evavold BD. Targeted loss of SHP1 in murine thymocytes dampens TCR signaling late in selection. Eur J Immunol. 2016;46:2103–2110. doi: 10.1002/eji.201646475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paster W, et al. GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. J Immunol. 2013;190:3749–3756. doi: 10.4049/jimmunol.1203389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi S, et al. THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat Immunol. 2017;18:433–441. doi: 10.1038/ni.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gascoigne NRJ, Brzostek J, Mehta M, Acuto O. SHP1-ing thymic selection. Eur J Immunol. 2016;46:2091–2094. doi: 10.1002/eji.201646582. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Shen K, Lu W, Cole PA. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J Biol Chem. 2003;278:4668–4674. doi: 10.1074/jbc.M210028200. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida K, Kharbanda S, Kufe D. Functional interaction between SHPTP1 and the Lyn tyrosine kinase in the apoptotic response to DNA damage. J Biol Chem. 1999;274:34663–34668. doi: 10.1074/jbc.274.49.34663. [DOI] [PubMed] [Google Scholar]
- 30.Poole AW, Jones ML. A SHPing tale: Perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal. 2005;17:1323–1332. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 32.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 33.Rosette C, et al. The impact of duration versus extent of TCR occupancy on T cell activation: A revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, et al. Crystal structure of human protein-tyrosine phosphatase SHP-1. J Biol Chem. 2003;278:6516–6520. doi: 10.1074/jbc.M210430200. [DOI] [PubMed] [Google Scholar]
- 35.Pani G, Colavitti R, Borrello S, Galeotti T. Endogenous oxygen radicals modulate protein tyrosine phosphorylation and JNK-1 activation in lectin-stimulated thymocytes. Biochem J. 2000;347:173–181. [PMC free article] [PubMed] [Google Scholar]
- 36.Hogquist KA, Xing Y, Hsu F-C, Shapiro VS. T cell adolescence: Maturation events beyond positive selection. J Immunol. 2015;195:1351–1357. doi: 10.4049/jimmunol.1501050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.