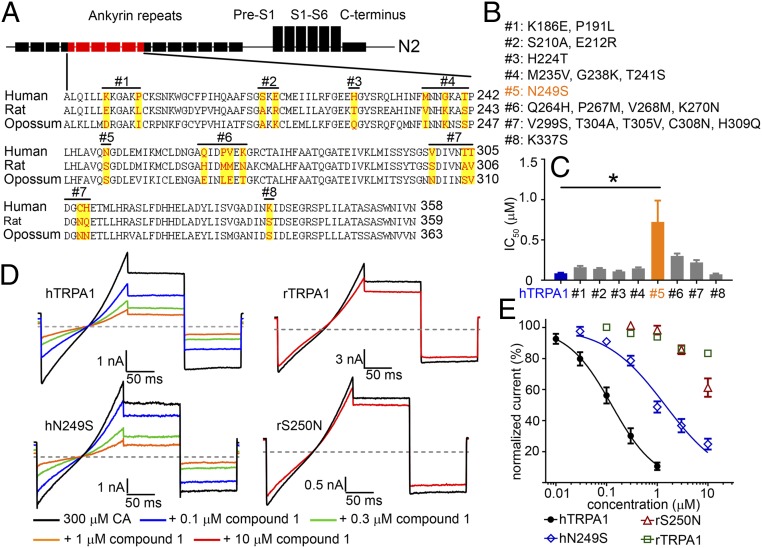
Fig. 3.
Residue N249 in hTRPA1 is critical but not sufficient for thiadiazole activity. (A) Sequence alignment among human, rat, and opossum TRPA1 within the N2 chimeric region. Residues targeted for site-directed mutagenesis are highlighted. hTRPA1 residues were mutated to rTRPA1 to produce eight mutant constructs as depicted above the sequence. (B) List of point mutation(s) for all eight constructs. (C) FLIPR IC50 of the mutant hTRPA1 constructs compared with WT hTRPA1 (n = 6 each group). Compound 1 was significantly less potent on Construct #5, IC50 = 0.72 ± 0.26 µM compared with hTRPA1, IC50 = 0.09 ± 0.01 µM (*P < 0.001 ANOVA with post hoc Dunnett’s t test). (D) Representative steady-state current traces showing Compound 1 inhibition of hN249S and rS250N constructs compared with WT hTRPA1 and rTRPA1 channels. Channels were activated with 300 µM cinnamaldehyde (CA) and Compound 1 was applied in the continued presence of agonist. The gray line denotes zero current level, and the voltage protocol is shown in Fig. 2A. (E) Concentration response curves of Compound 1 from manual patch-clamp electrophysiology. Steady-state current amplitudes were taken from the mean of the −40 mV step and normalized to 300 μM CA control. Each data point represents the normalized mean ± SEM current for the tested concentration. Potencies with 95% confidence intervals (n = 3 to 4 per construct): hTRPA1 IC50 = 0.13 µM (95% CI = 0.10–0.16 µM), hN249S IC50 = 1.43 µM (95% CI = 1.11–1.86 µM), rS250N IC50 > 10 µM, and rTRPA1 IC50 > 10 µM.